
The geometry of $I_3^ - $ is:
A. Triangular
B. Linear
C. Tetrahedral
D. T-shape
Answer
561k+ views
Hint: The triiodide ion contains three iodine atoms and are bonded two each other by two bond pairs and each iodine atom contains three lone pairs of electrons and overall charge is -1. The atoms are placed at 180 degrees from each other.
Complete step by step answer:
The given ion $I_3^ - $ is chemically known as triiodide which is referred to as triiodide ion. The triiodide ion is an anion with a negative charge. It is a polyhalogen ion which is formed by three iodide atoms. The triiodide ion is prepared by combining aqueous solution of iodide salt and iodine.
The reaction for the formation of triiodide is shown below.
${I_2} + {I^ - } \rightleftharpoons I_3^ - $
In this reaction iodide act as the Lewis base (electron donor) and iodine act as the Lewis acid (electron acceptor).
During the combination of iodine atoms, the central iodine gains a negative charge with value 1.
The structure of triiodide is shown below.
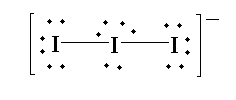
According to VSEPR theory, the central iodide atom contains three lone pairs of electrons. The terminal iodine is bonded axially to the central iodide atom in linear fashion due to the presence of three electron pairs. Thus the shape of the triiodide is linear and is symmetrical. The hybridization of $I_3^ - $is $s{p^3}d$. The bond angle between the atoms is 180 degrees.
Thus, the geometry of $I_3^ - $ is linear.
Therefore, the correct option is B.
Note:
The hybridization of the compound or ion is calculated by the formula as shown below.
$\dfrac{{Number\;of\;valence\;electrons + monovalent + (negative\;ch\arg e) - (positive\;ch\arg e)}}{2}$
In, $I_3^ - $ the number of valence electrons are 7
To find out the hybridization, place the values in the formula as shown below.
$ \Rightarrow \dfrac{{7 + 1 + 2}}{2}$
$ \Rightarrow \dfrac{{10}}{2}$
$ \Rightarrow 5$
The hybridization number is 5 which means the hybridization is $s{p^3}d$.
Complete step by step answer:
The given ion $I_3^ - $ is chemically known as triiodide which is referred to as triiodide ion. The triiodide ion is an anion with a negative charge. It is a polyhalogen ion which is formed by three iodide atoms. The triiodide ion is prepared by combining aqueous solution of iodide salt and iodine.
The reaction for the formation of triiodide is shown below.
${I_2} + {I^ - } \rightleftharpoons I_3^ - $
In this reaction iodide act as the Lewis base (electron donor) and iodine act as the Lewis acid (electron acceptor).
During the combination of iodine atoms, the central iodine gains a negative charge with value 1.
The structure of triiodide is shown below.

According to VSEPR theory, the central iodide atom contains three lone pairs of electrons. The terminal iodine is bonded axially to the central iodide atom in linear fashion due to the presence of three electron pairs. Thus the shape of the triiodide is linear and is symmetrical. The hybridization of $I_3^ - $is $s{p^3}d$. The bond angle between the atoms is 180 degrees.
Thus, the geometry of $I_3^ - $ is linear.
Therefore, the correct option is B.
Note:
The hybridization of the compound or ion is calculated by the formula as shown below.
$\dfrac{{Number\;of\;valence\;electrons + monovalent + (negative\;ch\arg e) - (positive\;ch\arg e)}}{2}$
In, $I_3^ - $ the number of valence electrons are 7
To find out the hybridization, place the values in the formula as shown below.
$ \Rightarrow \dfrac{{7 + 1 + 2}}{2}$
$ \Rightarrow \dfrac{{10}}{2}$
$ \Rightarrow 5$
The hybridization number is 5 which means the hybridization is $s{p^3}d$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




