
The factors that influences the ionization energies are:
A.The Size of atom
B.The charge on the nucleus
C.How effectively the inner electrons shell screen the nuclear charge
D.All of the above
Answer
583.2k+ views
Hint: Ionization energy of an element Is the amount of energy required to remove an electron from the outermost shell of an atom to form a positive ion. Ionization energy varies along a group and period and it is depending upon several factors.
Complete step by step answer:
The amount of energy required to remove an electron from the outermost shell of an isolated gaseous atom of an element is known as Ionization energy. The removal of an electron from the outermost shell results in the formation of a positive ion known as Cation.
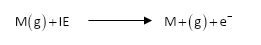
Ionization energy depends upon several factors. One is the atomic size of an atom. The larger the atomic size, the smaller will be the ionization energy. The reason is that as the size of the atom increases, outer electrons become far away from the nucleus. Hence the attraction between nucleus and outer electrons decreases and that electron can be easily removed.
Ionization energy also depends upon the nuclear charge of an atom. As the attraction between nucleus and outermost electrons increases it becomes difficult to remove that electron, thus ionization energy increases on increasing nuclear charge of an atom.
Ionization energy depends upon the number of electrons in the inner shells. As the number of inner electrons increase, Ionization energy decreases. Because, those electrons act as a screen between the nucleus and the electrons in the outermost shell. This effect is known as screening effect or shielding effect. As the number of inner electrons increases greater will be the screening effect. Consequently, electrons in the valence shell experience less attraction from the nucleus. So, Ionization energy will be low.
The other factors which affect Ionization energy are Electronic configuration, the effect of removal of s, p, d, f electrons from the same energy. S electrons are present near the nucleus as compared to p, d, f electrons. So, it has more penetrating power. S electrons experience more attraction from the nucleus than p, d, f electrons.
Certain electronic configurations are more stable than others. Half- filled and Full- filled electronic configuration has high Ionization energy. Because those are stable configurations. It's difficult to remove electrons from a stable configuration.
Hence, the answer is option (D) i.e All of the above.
Note: If one electron is removed from an atom, then it is difficult to remove the second electron. This is because on removing an electron the number of electrons decreases but the nuclear charge remains the same. Hence, the second and subsequent removal of electrons require more energy. Thus, Ionization energy increases.
Complete step by step answer:
The amount of energy required to remove an electron from the outermost shell of an isolated gaseous atom of an element is known as Ionization energy. The removal of an electron from the outermost shell results in the formation of a positive ion known as Cation.

Ionization energy depends upon several factors. One is the atomic size of an atom. The larger the atomic size, the smaller will be the ionization energy. The reason is that as the size of the atom increases, outer electrons become far away from the nucleus. Hence the attraction between nucleus and outer electrons decreases and that electron can be easily removed.
Ionization energy also depends upon the nuclear charge of an atom. As the attraction between nucleus and outermost electrons increases it becomes difficult to remove that electron, thus ionization energy increases on increasing nuclear charge of an atom.
Ionization energy depends upon the number of electrons in the inner shells. As the number of inner electrons increase, Ionization energy decreases. Because, those electrons act as a screen between the nucleus and the electrons in the outermost shell. This effect is known as screening effect or shielding effect. As the number of inner electrons increases greater will be the screening effect. Consequently, electrons in the valence shell experience less attraction from the nucleus. So, Ionization energy will be low.
The other factors which affect Ionization energy are Electronic configuration, the effect of removal of s, p, d, f electrons from the same energy. S electrons are present near the nucleus as compared to p, d, f electrons. So, it has more penetrating power. S electrons experience more attraction from the nucleus than p, d, f electrons.
Certain electronic configurations are more stable than others. Half- filled and Full- filled electronic configuration has high Ionization energy. Because those are stable configurations. It's difficult to remove electrons from a stable configuration.
Hence, the answer is option (D) i.e All of the above.
Note: If one electron is removed from an atom, then it is difficult to remove the second electron. This is because on removing an electron the number of electrons decreases but the nuclear charge remains the same. Hence, the second and subsequent removal of electrons require more energy. Thus, Ionization energy increases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




