
The e/m ratio for electron was determined by:
A. JJ Thomson
B. Planck
C. Einstein
D. Schrodinger
Answer
592.8k+ views
Hint: According to classical electrodynamics, the value of the mass-to - charge ratio is that, when exposed to the same electric and magnetic fields, two particles with the same mass-to - charge ratio travel along the same direction in a vacuum. Its SI unit is kg / C.
Complete answer:
In the nineteenth century, J.J Thomson proposed Thomson’s Atomic Model discovered the electron to mark inception to the planet of subatomic particles. Once the electron was discovered, he continued his experiments to calculate the charge and therefore the mass of the electron. With the assistance of his experiments, he derived a formula for the calculation of charge to mass ratio of the electron.
The charge to mass ratio of the electron is given by:
${e}/{m=1.758820\times }\;{{10}^{11}}{C}/{kg}\;$
Where,
m = mass of an electron in kg = $9.10938356\times {{10}^{-31}}$ kg.
e = magnitude of the charge of an electron in coulombs = $1.602\times {{10}^{-19}}$ coulombs.
Experimental setup for the determination of charge to mass ratio of the electron.
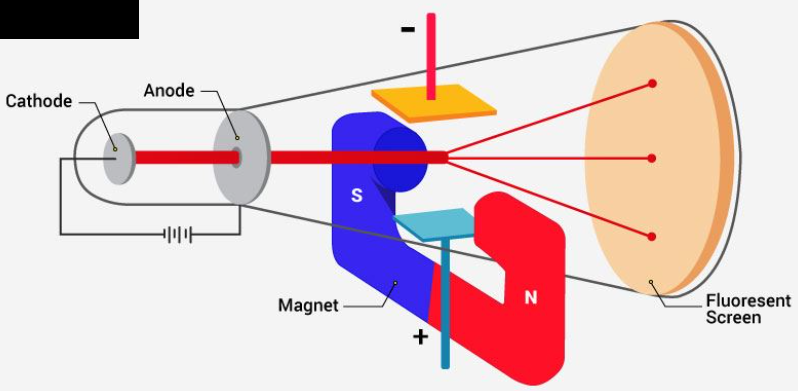
While completing the discharge tube experiment, Thomson observed that the particles of the cathode deviate from their path. He noticed the amount of deviation within the presence of an electrical or magnetic field depends on various related parameters. They are:
Particles with a greater magnitude of the charge experienced greater interaction with the electrical or magnetic field. Thus, they exhibited greater deflection.
Lighter particles experienced greater deflection. Thus, deflection is inversely proportional to the mass of the particle.
Deflection of the particle from their path is directly proportional to the strength of the electrical and therefore the magnetic flux present.
Let us now understand this with the assistance of his experimental observations.
The electrons deviated from their path and hit the cathode ray tube at point ‘x’ within the presence of a known field.
Similarly, an electron strikes the discharge tube at point ‘z’ when only the magnetic flux is present.
Thus, to form electrons continue on an equivalent path we'd like to balance the electrical and magnetic flux working on them.
Finally, based on the deflection of the electron, Thomson calculated the worth of charge to mass ratio of the electron.
Hence, the e/m ratio for electrons was determined by JJ Thomson.
So, the correct answer is “Option A”.
Note: Always remember, the charge to mass ratio of the electron is given by:
${e}/{m=1.758820\times }\;{{10}^{11}}{C}/{kg}\;$
Mass of an electron (m) in kg = $9.10938356\times {{10}^{-31}}$ kg.
Magnitude of the charge of an electron (e) in coulombs = $1.602\times {{10}^{-19}}$ coulombs.
The electron is a low-mass, negatively charged particle. It can easily be deflected by passing close to others electrons or the positive nucleus of an atom.
Complete answer:
In the nineteenth century, J.J Thomson proposed Thomson’s Atomic Model discovered the electron to mark inception to the planet of subatomic particles. Once the electron was discovered, he continued his experiments to calculate the charge and therefore the mass of the electron. With the assistance of his experiments, he derived a formula for the calculation of charge to mass ratio of the electron.
The charge to mass ratio of the electron is given by:
${e}/{m=1.758820\times }\;{{10}^{11}}{C}/{kg}\;$
Where,
m = mass of an electron in kg = $9.10938356\times {{10}^{-31}}$ kg.
e = magnitude of the charge of an electron in coulombs = $1.602\times {{10}^{-19}}$ coulombs.
Experimental setup for the determination of charge to mass ratio of the electron.

While completing the discharge tube experiment, Thomson observed that the particles of the cathode deviate from their path. He noticed the amount of deviation within the presence of an electrical or magnetic field depends on various related parameters. They are:
Particles with a greater magnitude of the charge experienced greater interaction with the electrical or magnetic field. Thus, they exhibited greater deflection.
Lighter particles experienced greater deflection. Thus, deflection is inversely proportional to the mass of the particle.
Deflection of the particle from their path is directly proportional to the strength of the electrical and therefore the magnetic flux present.
Let us now understand this with the assistance of his experimental observations.
The electrons deviated from their path and hit the cathode ray tube at point ‘x’ within the presence of a known field.
Similarly, an electron strikes the discharge tube at point ‘z’ when only the magnetic flux is present.
Thus, to form electrons continue on an equivalent path we'd like to balance the electrical and magnetic flux working on them.
Finally, based on the deflection of the electron, Thomson calculated the worth of charge to mass ratio of the electron.
Hence, the e/m ratio for electrons was determined by JJ Thomson.
So, the correct answer is “Option A”.
Note: Always remember, the charge to mass ratio of the electron is given by:
${e}/{m=1.758820\times }\;{{10}^{11}}{C}/{kg}\;$
Mass of an electron (m) in kg = $9.10938356\times {{10}^{-31}}$ kg.
Magnitude of the charge of an electron (e) in coulombs = $1.602\times {{10}^{-19}}$ coulombs.
The electron is a low-mass, negatively charged particle. It can easily be deflected by passing close to others electrons or the positive nucleus of an atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




