
The electron configuration for chromium is $\left[ Ar \right]4{{s}^{1}}\text{ }3{{d}^{5}}$. Select the best explanation for this irregular electron configuration.
A. A half-filled d orbital is slightly lower in energy than the 4s, thus filling first.
B. The 4s orbital is at a lower energy than the 3d orbital, thus filling first.
C. Chromium is a transition metal and its electrons are loosely held.
D. d orbitals are dominant and shield electrons from orbitals close in energy.
Answer
592.5k+ views
Hint: According to Hund’s principle which states that, when electron starts filling up subshells, they do it such that the electrons of the same spin must solely occupy the orbitals within the subshell first and then the electrons of opposite spin will start filling up the remaining space in the orbitals.
Complete answer:
We know that the order of stability is defined as:
Fully-filled orbital $>$ half-filled orbital $>$ partially-filled orbital
We also know that s-orbital can accommodate a maximum of 2 electrons, p-orbital can accommodate a maximum of 6 electrons, d-orbital can accommodate a maximum of 10 electrons and f-orbital can accommodate a maximum of 14 electrons.
So, in $4{{s}^{1}}\text{ }3{{d}^{5}}$we see that both the s and p orbitals are half-filled. In case of chromium, the expected electronic configuration is $4{{s}^{2}}\text{ }3{{d}^{4}}$, yet actually we see that one electron from 4s gets transferred to 3d orbital making it $4{{s}^{1}}\text{ }3{{d}^{5}}$. This happens because $4{{s}^{1}}\text{ }3{{d}^{5}}$ being a more stable configuration.
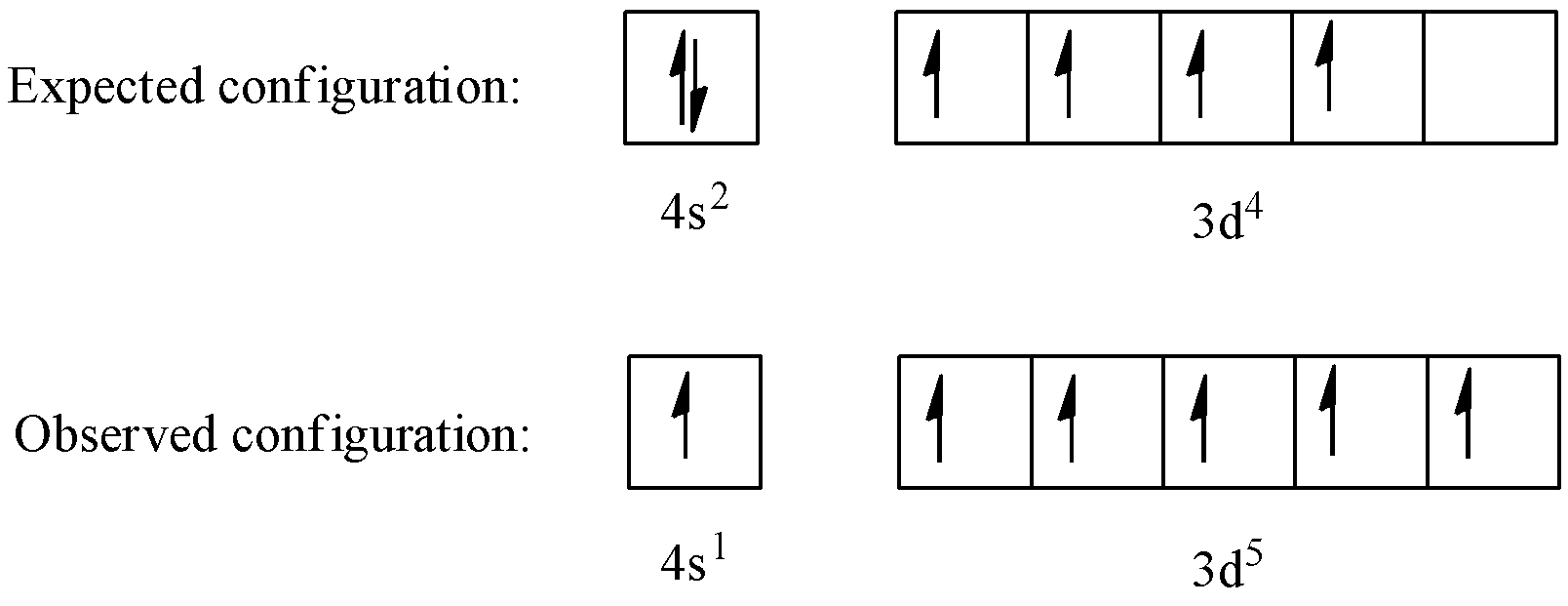
Electron orbitals are most stable when fully-filled or half-filled, hence the most stable configuration of electrons for 3d subshells is either $3{{d}^{10}}$or $3{{d}^{5}}$. In the case of chromium, after $4{{s}^{2}}\text{ }3{{d}^{4}}$ configuration is attained, and electron from s-orbital gets transferred to 3d subshell because $3{{d}^{5}}$ is much more stable configuration than $3{{d}^{4}}$. This is why the configuration for chromium is $4{{s}^{1}}\text{ }3{{d}^{5}}$.
So, the correct answer is “Option A”.
Note: In the case of chromium, it is an exception of the Aufbau principle and the systematic rule of the principle does not comply with its electron configuration. The classical rule that there are orbitals with different energy levels state that
$1s\text{ }<\text{ }2s\text{ }<\text{ }2p\text{ }<\text{ }3s\text{ }<\text{ }3p\text{ }<\text{ }4s\text{ }<\text{ }3d\text{ }<\text{ }4p$ and so on. Same results are seen in $Cu$ where the expected configuration is $[Ar]4{{s}^{2}}3{{d}^{9}}$ but the observed configuration is $[Ar]4{{s}^{1}}3{{d}^{10}}$
Complete answer:
We know that the order of stability is defined as:
Fully-filled orbital $>$ half-filled orbital $>$ partially-filled orbital
We also know that s-orbital can accommodate a maximum of 2 electrons, p-orbital can accommodate a maximum of 6 electrons, d-orbital can accommodate a maximum of 10 electrons and f-orbital can accommodate a maximum of 14 electrons.
So, in $4{{s}^{1}}\text{ }3{{d}^{5}}$we see that both the s and p orbitals are half-filled. In case of chromium, the expected electronic configuration is $4{{s}^{2}}\text{ }3{{d}^{4}}$, yet actually we see that one electron from 4s gets transferred to 3d orbital making it $4{{s}^{1}}\text{ }3{{d}^{5}}$. This happens because $4{{s}^{1}}\text{ }3{{d}^{5}}$ being a more stable configuration.

Electron orbitals are most stable when fully-filled or half-filled, hence the most stable configuration of electrons for 3d subshells is either $3{{d}^{10}}$or $3{{d}^{5}}$. In the case of chromium, after $4{{s}^{2}}\text{ }3{{d}^{4}}$ configuration is attained, and electron from s-orbital gets transferred to 3d subshell because $3{{d}^{5}}$ is much more stable configuration than $3{{d}^{4}}$. This is why the configuration for chromium is $4{{s}^{1}}\text{ }3{{d}^{5}}$.
So, the correct answer is “Option A”.
Note: In the case of chromium, it is an exception of the Aufbau principle and the systematic rule of the principle does not comply with its electron configuration. The classical rule that there are orbitals with different energy levels state that
$1s\text{ }<\text{ }2s\text{ }<\text{ }2p\text{ }<\text{ }3s\text{ }<\text{ }3p\text{ }<\text{ }4s\text{ }<\text{ }3d\text{ }<\text{ }4p$ and so on. Same results are seen in $Cu$ where the expected configuration is $[Ar]4{{s}^{2}}3{{d}^{9}}$ but the observed configuration is $[Ar]4{{s}^{1}}3{{d}^{10}}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




