
The difference in the oxidation numbers of the two types of sulfur atoms in
\[N{a_2}{S_4}{0_6}\] is:
A. 5
B. 4
C. 3
D. 6
Answer
582.6k+ views
Hint: To solve this question, you must follow these steps: First, draw the molecular structure of \[N{a_2}{S_4}{0_6}\] Based on this diagram, solve for each option given. Only one of these options is right and upon detailed observation, you would be able to find it.
Complete step by step answer:
Before we move forward with the solution of this question, let us draw the molecular structure of thiosulphuric acid:
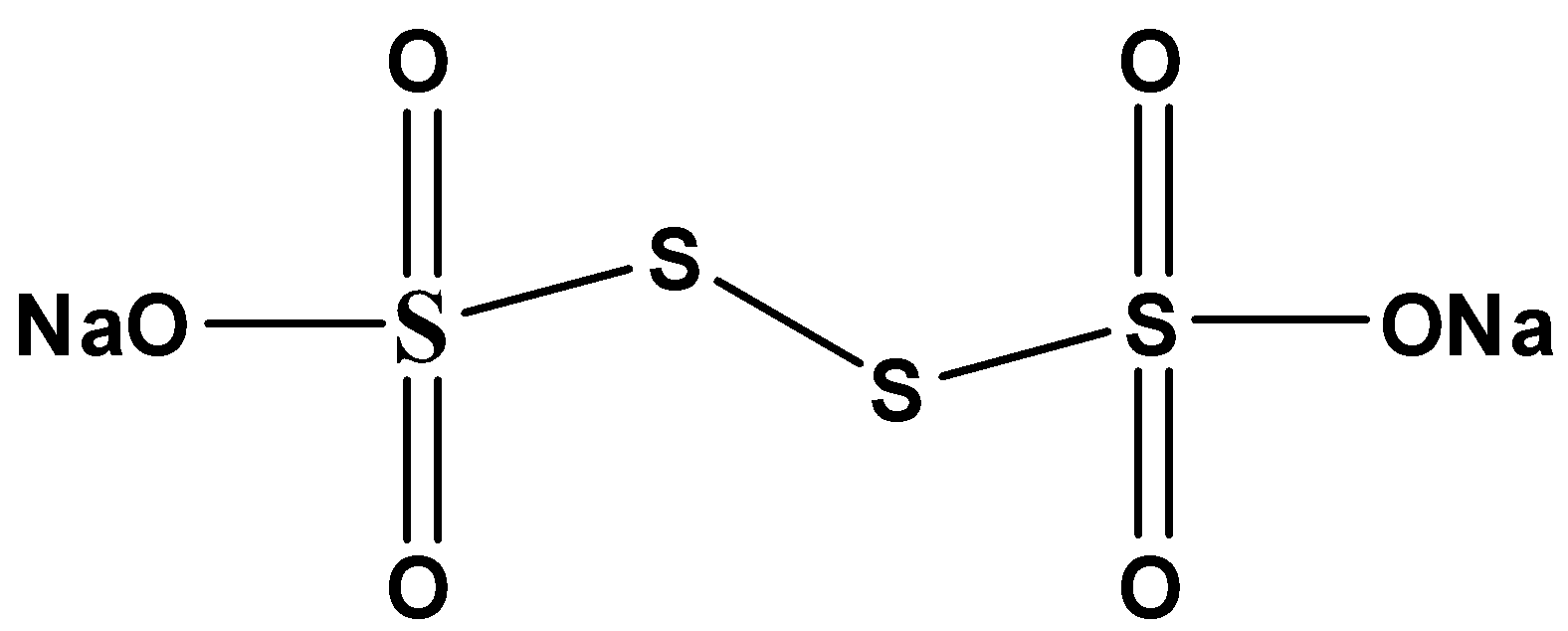
Now, to determine the correct statement about thiosulphuric acid from the given options, let us discuss them:
1.Each sulphur atom is in an identical oxidation state:
The terminal sulphur atoms have formed 4 sigma bonds and 2 pi bonds with the neighbouring atoms. On the other hand, the other sulphur atoms have formed only one sigma. Hence the oxidation states of both the sulphur atoms are different.
2.There is an \[S - S\] linkage present:
As we can see in the molecular structure, there is indeed a single bond present between the two sulphur atoms.
3.The middle sulphur atoms are in 0 and terminal sulphur atoms are in +5 oxidation state:
The electronic configuration of sulphur is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}\] . Now, from point 2, we can say that the terminal sulphur shares with all the 6 electrons present in the valence shell and achieves the +6-oxidation state. While the other sulphur atom accepts the 2 electrons shared by the neighbour sulphur atom.
So, considering this information, the sum of the oxidation states of sulfur atoms is,
(5+0) =5
So, the correct option is, A.
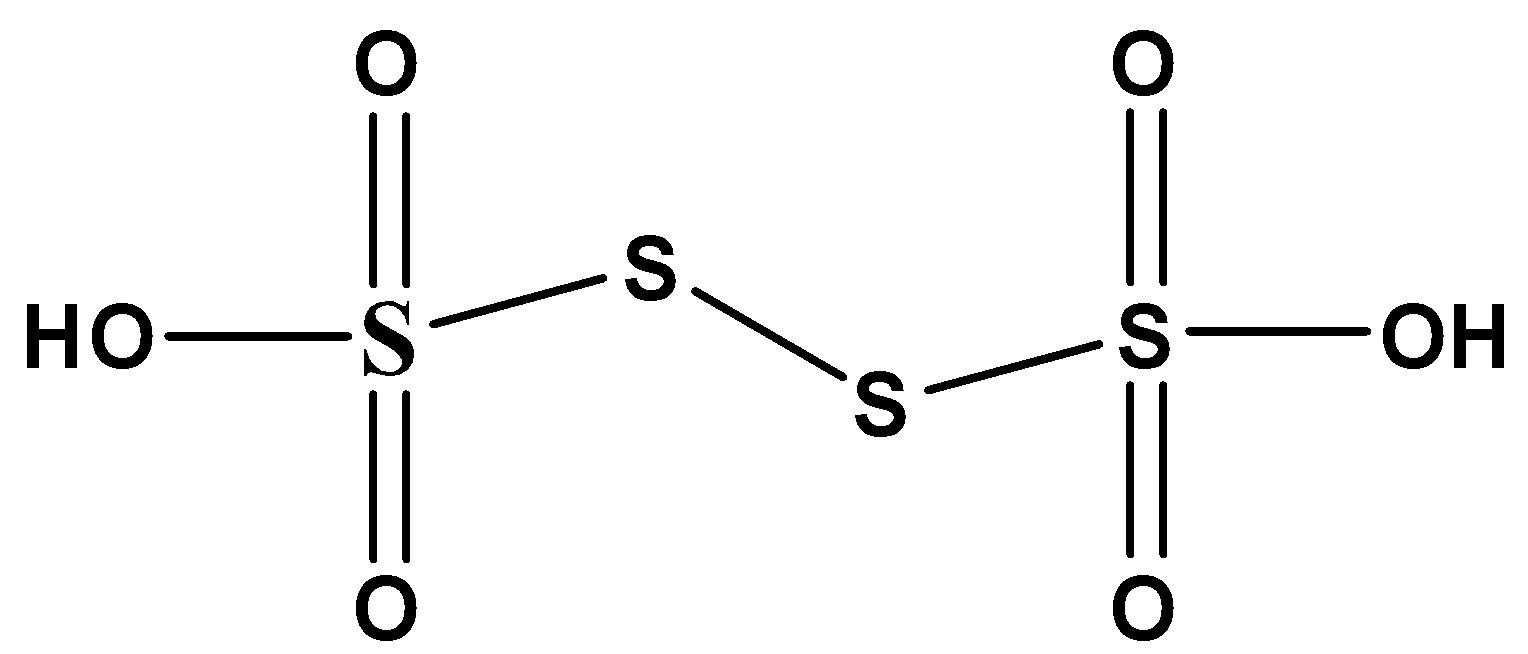
Note:This is a salt of a sulfur oxoacid. The acid cannot be made by acidifying aqueous salt solutions as the acid readily decomposes in water. There are 2 \[ - OH\] groups present in that acid compound. Each \[ - OH\] group has one replaceable hydrogen. Hence that acid has 2 replaceable hydrogens. The structure of that acid as follows,
Complete step by step answer:
Before we move forward with the solution of this question, let us draw the molecular structure of thiosulphuric acid:

Now, to determine the correct statement about thiosulphuric acid from the given options, let us discuss them:
1.Each sulphur atom is in an identical oxidation state:
The terminal sulphur atoms have formed 4 sigma bonds and 2 pi bonds with the neighbouring atoms. On the other hand, the other sulphur atoms have formed only one sigma. Hence the oxidation states of both the sulphur atoms are different.
2.There is an \[S - S\] linkage present:
As we can see in the molecular structure, there is indeed a single bond present between the two sulphur atoms.
3.The middle sulphur atoms are in 0 and terminal sulphur atoms are in +5 oxidation state:
The electronic configuration of sulphur is \[1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}\] . Now, from point 2, we can say that the terminal sulphur shares with all the 6 electrons present in the valence shell and achieves the +6-oxidation state. While the other sulphur atom accepts the 2 electrons shared by the neighbour sulphur atom.
So, considering this information, the sum of the oxidation states of sulfur atoms is,
(5+0) =5
So, the correct option is, A.
Note:This is a salt of a sulfur oxoacid. The acid cannot be made by acidifying aqueous salt solutions as the acid readily decomposes in water. There are 2 \[ - OH\] groups present in that acid compound. Each \[ - OH\] group has one replaceable hydrogen. Hence that acid has 2 replaceable hydrogens. The structure of that acid as follows,

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




