
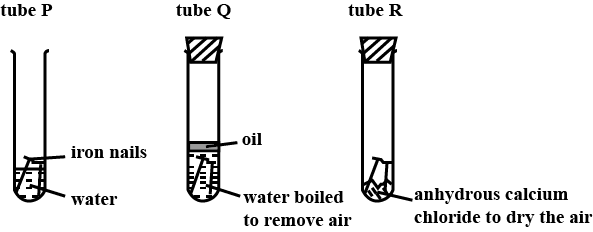
The diagram shows experiments involving the rusting of iron.
The following results were suggested
1. In tube P, the iron nails rust
2. In tube Q, the iron nails do not rust
3. In tube R, the iron nails do not rust
Which results are correct?
A. 1 and 2 only
B. 1 and 3 only
C. 2 and 3 only
D. 1, 2 and 3

Answer
560.4k+ views
Hint: Iron is a chemical element represented by Fe which Fe stands for ferrum which is a Latin word and the atomic number of Fe is 26. This metal belongs to group 8 of the periodic table and kept in the category of transition elements because it contains d subshell.
Complete step by step answer:
- Rusting generally acts on the surface of iron due to which the surface becomes dull. This can be explained by the most reactive nature of iron. Iron reacts with oxygen present in air and forms oxides due to which it gets corroded. The conditions necessary for rust are water and oxygen present in the environment.
- In the given cases tube P is open so it has both conditions of rusting water as well as environmental oxygen so the nail present in tube P will develop rust, In case Q we can see that tube is covered with cork so no oxygen is present therefore iron will not develop rust and the nail in tube Q do not rust similarly tube R is also closed therefore no supply of oxygen and the nail in R also not produce any rust. The correct answer is option “D” .
Note: Some metallic objects develop a change on their surface with the passage of time or we can say that they are kept unused for a long time this change is due to the chemical reactions proceeding by them in the environment and this process is known as corrosion.
Complete step by step answer:
- Rusting generally acts on the surface of iron due to which the surface becomes dull. This can be explained by the most reactive nature of iron. Iron reacts with oxygen present in air and forms oxides due to which it gets corroded. The conditions necessary for rust are water and oxygen present in the environment.
- In the given cases tube P is open so it has both conditions of rusting water as well as environmental oxygen so the nail present in tube P will develop rust, In case Q we can see that tube is covered with cork so no oxygen is present therefore iron will not develop rust and the nail in tube Q do not rust similarly tube R is also closed therefore no supply of oxygen and the nail in R also not produce any rust. The correct answer is option “D” .
Note: Some metallic objects develop a change on their surface with the passage of time or we can say that they are kept unused for a long time this change is due to the chemical reactions proceeding by them in the environment and this process is known as corrosion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




