
The correct structure of ethylenediaminetetraacetic acid (EDTA) is




Answer
590.7k+ views
Hint: Ethylene diamine tetra acetic acid or the $\text{EDTA}$ is a tetraprotic acid. The structure of it consists of the ethane with two amine groups attached to it forming an ethylene diamine. Each of the hydrogen on the amine group is replaced by the acetic acid $\text{(-C}{{\text{H}}_{\text{2}}}\text{COOH)}$ group. The resultant structures are the$\text{EDTA}$. It is a complexing agent.
Complete answer:
Ethylene diamine tetra acetic acid or $\text{EDTA}$ is a complexing agent.
$\text{EDTA}$ Was first synthesized by Ferdinand Munz in 1935. This $\text{EDTA}$ is also called the amino polycarboxylic acid. It is colourless and water soluble in its salt form.
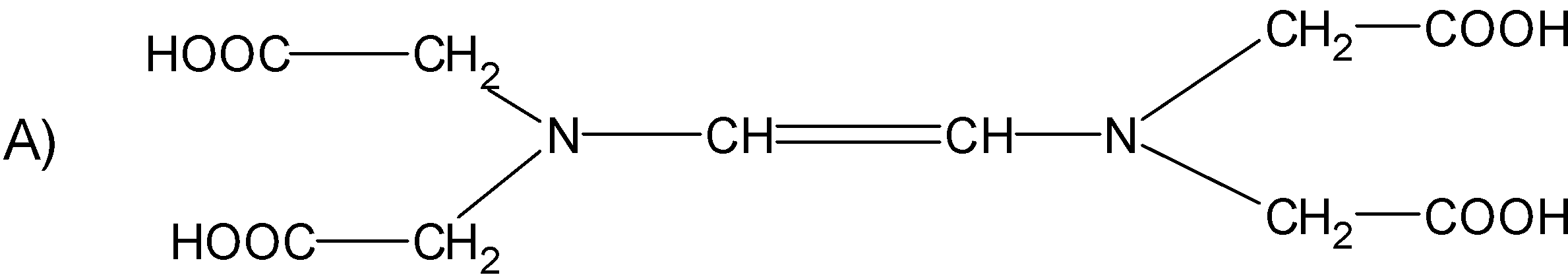
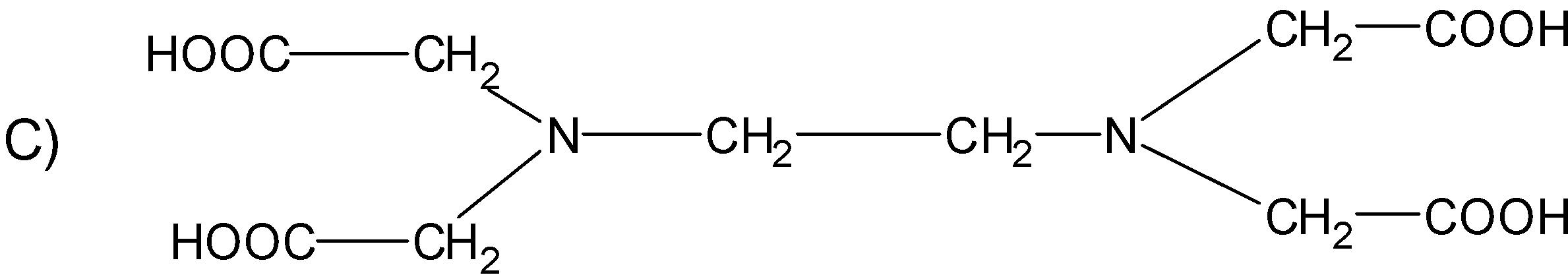
The structure $\text{EDTA}$ consists of the basic ethane group which is attached with the two $\text{-N}{{\text{H}}_{\text{2}}}$ groups at the terminal positions forming an ethylene diamine. The two hydrogens from each amine group situated at the terminal positions are replaced by the acetic acid group$\text{(-C}{{\text{H}}_{\text{2}}}\text{COO)}$. There are a total of four acetic acid groups attached to the ethylene diamine.
The structure for the ethylene diamine tetra acetic acid or $\text{EDTA}$ is as follows.
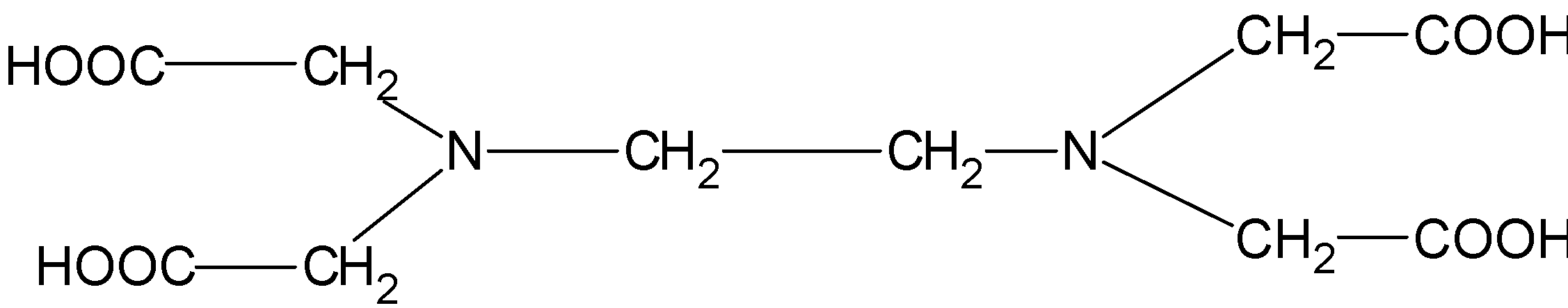
$\text{EDTA}$ is a hexadentate ligand.
It has four carboxylic acid groups and two amine groups with lone pairs of electrons on it. It is polyprotic acid.
Along with the four carboxylic acid groups $\text{EDTA}$ can add two more hydrogens on the two nitrogens of the amine groups.
Hence, (C) is the correct option.
Additional information:
The $\text{EDTA}$ has the special ability to form a complex with the metals. It forms the $1:1$metal-$\text{EDTA}$ complexes. $\text{EDTA}$ Binds with the metal ions to form a soluble complex of metal.
As $\text{EDTA}$ can donate four electrons the complex formed by the metal and $\text{EDTA}$ is as shown below:
${{\text{M}}^{\text{+n}}}\text{+}{{\text{Y}}^{\text{-4}}}\to \text{M}{{\text{Y}}^{\text{n-4}}}$
Where M is the metal and y is the complexing agent $\text{EDTA}$.
Metal analysis can be done using the titration of $\text{EDTA}$ the metal using the metal ion indicators. One of the famous and most common uses of $\text{EDTA}$is to determine the hardness of the water.
$\text{C}{{\text{a}}^{\text{2+}}}\text{+N}{{\text{a}}_{\text{2}}}\text{-EDTA}\rightleftarrows \text{Ca-EDTA+2N}{{\text{a}}^{\text{+}}}$
It is usually present as a disodium salt of $\text{EDTA}$ which is readily soluble in water.
Note:
$\text{EDTA}$is an abbreviation used for the Ethylene Diamine Tetra-Acetic acid. It is acid thus in the acidic medium it is fully protonated. Hence most of the metal-$\text{EDTA}$ reactions favours in slightly basic or basic conditions.
Complete answer:
Ethylene diamine tetra acetic acid or $\text{EDTA}$ is a complexing agent.
$\text{EDTA}$ Was first synthesized by Ferdinand Munz in 1935. This $\text{EDTA}$ is also called the amino polycarboxylic acid. It is colourless and water soluble in its salt form.
The structure $\text{EDTA}$ consists of the basic ethane group which is attached with the two $\text{-N}{{\text{H}}_{\text{2}}}$ groups at the terminal positions forming an ethylene diamine. The two hydrogens from each amine group situated at the terminal positions are replaced by the acetic acid group$\text{(-C}{{\text{H}}_{\text{2}}}\text{COO)}$. There are a total of four acetic acid groups attached to the ethylene diamine.
The structure for the ethylene diamine tetra acetic acid or $\text{EDTA}$ is as follows.

$\text{EDTA}$ is a hexadentate ligand.
It has four carboxylic acid groups and two amine groups with lone pairs of electrons on it. It is polyprotic acid.
Along with the four carboxylic acid groups $\text{EDTA}$ can add two more hydrogens on the two nitrogens of the amine groups.
Hence, (C) is the correct option.
Additional information:
The $\text{EDTA}$ has the special ability to form a complex with the metals. It forms the $1:1$metal-$\text{EDTA}$ complexes. $\text{EDTA}$ Binds with the metal ions to form a soluble complex of metal.
As $\text{EDTA}$ can donate four electrons the complex formed by the metal and $\text{EDTA}$ is as shown below:
${{\text{M}}^{\text{+n}}}\text{+}{{\text{Y}}^{\text{-4}}}\to \text{M}{{\text{Y}}^{\text{n-4}}}$
Where M is the metal and y is the complexing agent $\text{EDTA}$.
Metal analysis can be done using the titration of $\text{EDTA}$ the metal using the metal ion indicators. One of the famous and most common uses of $\text{EDTA}$is to determine the hardness of the water.
$\text{C}{{\text{a}}^{\text{2+}}}\text{+N}{{\text{a}}_{\text{2}}}\text{-EDTA}\rightleftarrows \text{Ca-EDTA+2N}{{\text{a}}^{\text{+}}}$
It is usually present as a disodium salt of $\text{EDTA}$ which is readily soluble in water.
Note:
$\text{EDTA}$is an abbreviation used for the Ethylene Diamine Tetra-Acetic acid. It is acid thus in the acidic medium it is fully protonated. Hence most of the metal-$\text{EDTA}$ reactions favours in slightly basic or basic conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




