
The correct sequence of decreasing number of pi-bonds in the structure of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}{\rm{,}}{{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}\;{\rm{and}}\;{{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}}$ is:
A. ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} > {{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}}$
B. ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}}$
C. ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} > {{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$
D. ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$
Answer
577.5k+ views
Hint: We know that the double bond is generally categorized as pi bond. In the chemical structure, the compound has three types of bonds: one is single bond, second is double bond and third is triple bond.
Complete answer
As we all know, the name of the compound ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$ is sulfurous acid. Generally, it is the tautomer of the acid named as sulfonic. The composition of sulfurous acid consists of two hydrogen atoms, three oxygen atoms along with one sulfur atom. It usually shows the property of the inorganic group. The structure of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$ is shown below.
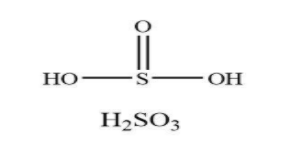
From the structure, it is clear that sulfurous acid has only one pi bond in the chemical structure.
Similarly, the name of the compound ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_4}$ is sulfuric acid. Generally, it is named as oil of vitriol. The composition of sulfuric acid consists of two hydrogen atoms, four oxygen atoms along with one sulfur atom. When it is dissolved in water it gives an exothermic reaction and produces large amounts of heat. The structure of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_4}$ is shown below.
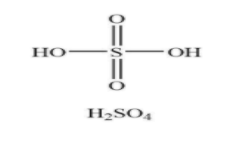
From the structure, it is clear that sulfuric acid has only two pi bonds in the chemical structure.
Similarly, the name of the compound ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_2}{{\rm{O}}_7}$ is disulfuric acid. Generally, it is named as oleum. The composition of disulfuric acid consists of two hydrogen atoms, seven oxygen atoms along with two sulfur atoms. The structure of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_4}$ is shown below.
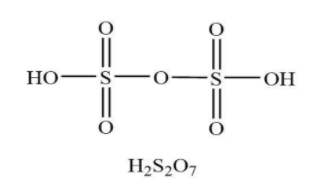
From the structure, it is clear that disulfuric acid has only four pi bonds in the chemical structure.
Thus, it clear that, the decreasing order of pi bonds in the given chemical compound is ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$.
Hence, the correct option for this given question is D that is ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$.
Note:
All the given compounds like sulfurous acid, sulfuric acid and disulfuric acid are strong acids and used in the laboratory reagent. Alternatively, for this question, one can think that as the number of oxygen atoms increases, the number of pi bonds also increases.
Complete answer
As we all know, the name of the compound ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$ is sulfurous acid. Generally, it is the tautomer of the acid named as sulfonic. The composition of sulfurous acid consists of two hydrogen atoms, three oxygen atoms along with one sulfur atom. It usually shows the property of the inorganic group. The structure of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$ is shown below.

From the structure, it is clear that sulfurous acid has only one pi bond in the chemical structure.
Similarly, the name of the compound ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_4}$ is sulfuric acid. Generally, it is named as oil of vitriol. The composition of sulfuric acid consists of two hydrogen atoms, four oxygen atoms along with one sulfur atom. When it is dissolved in water it gives an exothermic reaction and produces large amounts of heat. The structure of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_4}$ is shown below.

From the structure, it is clear that sulfuric acid has only two pi bonds in the chemical structure.
Similarly, the name of the compound ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_2}{{\rm{O}}_7}$ is disulfuric acid. Generally, it is named as oleum. The composition of disulfuric acid consists of two hydrogen atoms, seven oxygen atoms along with two sulfur atoms. The structure of ${{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_4}$ is shown below.

From the structure, it is clear that disulfuric acid has only four pi bonds in the chemical structure.
Thus, it clear that, the decreasing order of pi bonds in the given chemical compound is ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$.
Hence, the correct option for this given question is D that is ${{\rm{H}}_{\rm{2}}}{{\rm{S}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{4}}} > {{\rm{H}}_{\rm{2}}}{\rm{S}}{{\rm{O}}_{\rm{3}}}$.
Note:
All the given compounds like sulfurous acid, sulfuric acid and disulfuric acid are strong acids and used in the laboratory reagent. Alternatively, for this question, one can think that as the number of oxygen atoms increases, the number of pi bonds also increases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




