
The correct order of decreasing dipole moment of (I) toluene, (II) m-dichlorobenzene, (III) o-dichlorobenzene and (IV) p-dichlorobenzene.
IV < II < I < III
IV < I < II < III
I < IV < II < III
IV < I < III < II
Answer
597k+ views
Hint- We know that the dipole moment occurs when there is a separation of charge in the complex. Dipole moments can occur between any two ions in either an ionic bond or in between the atoms in a covalent bond.
Complete step by step answer:
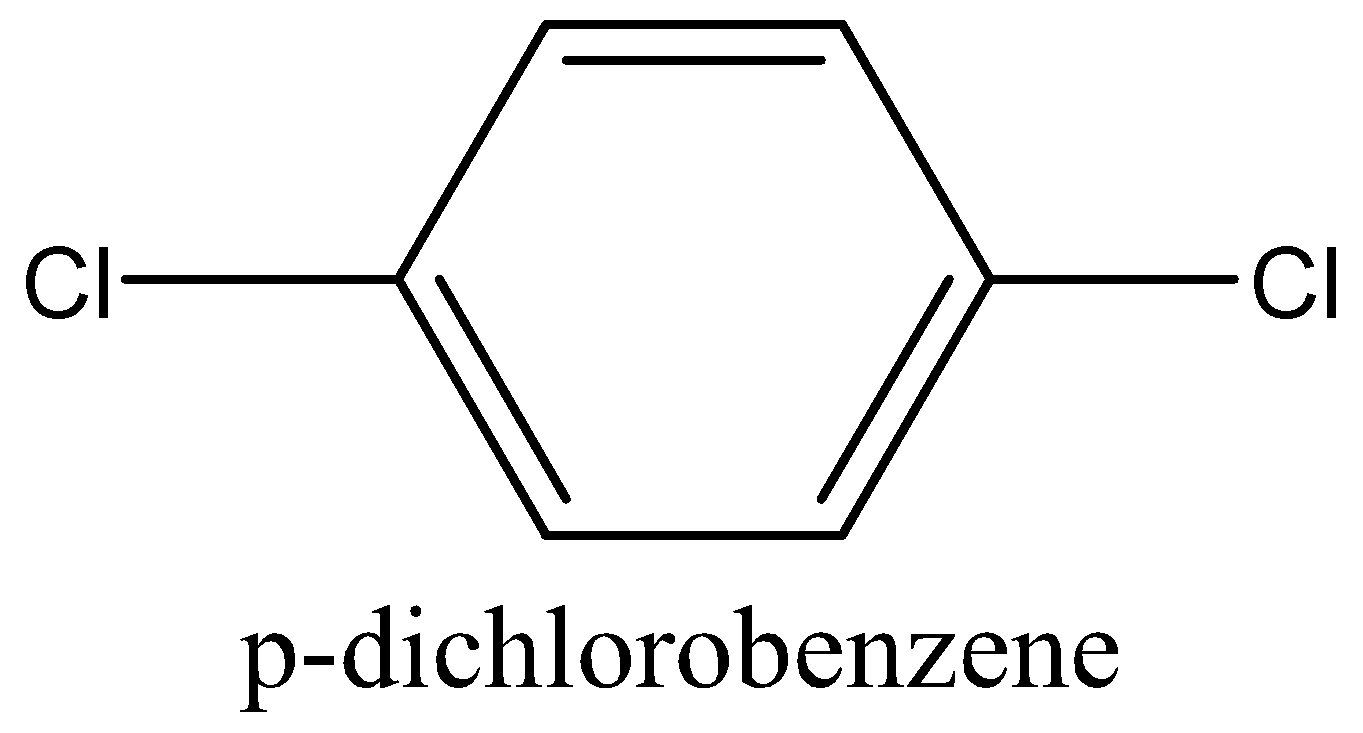
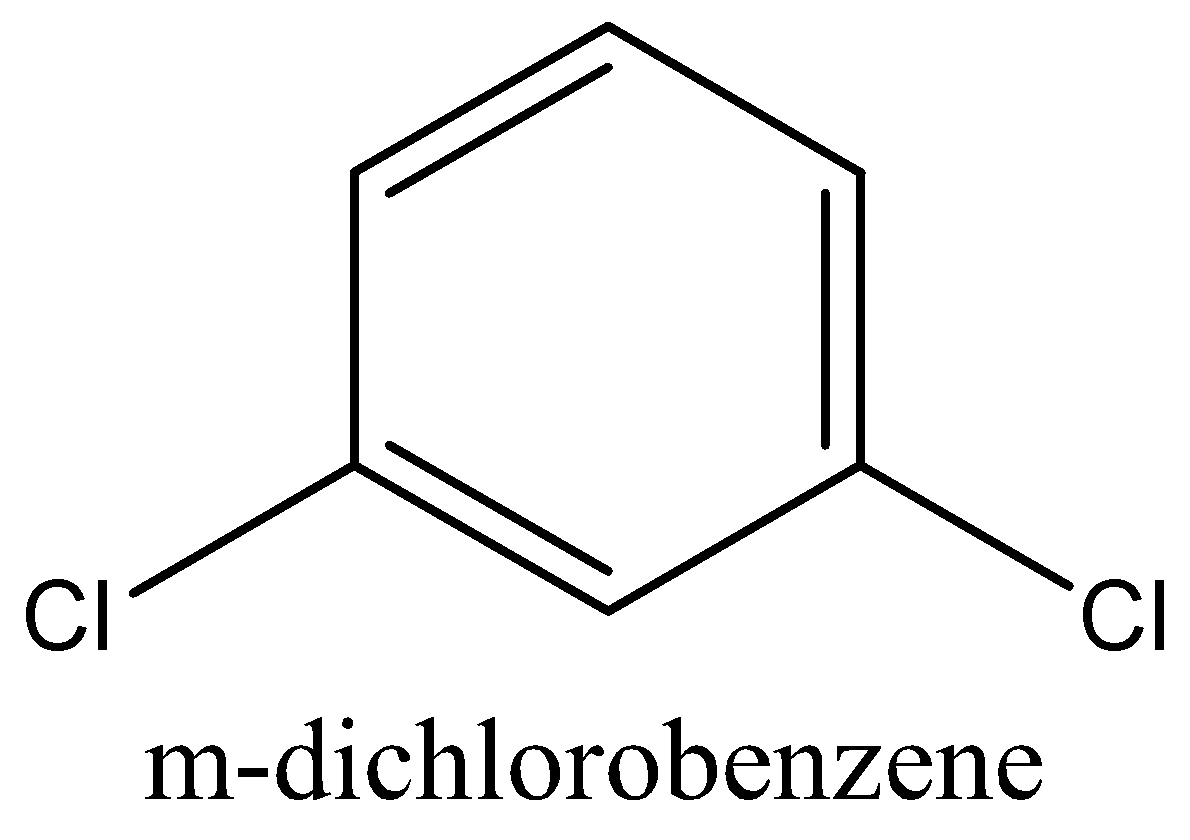
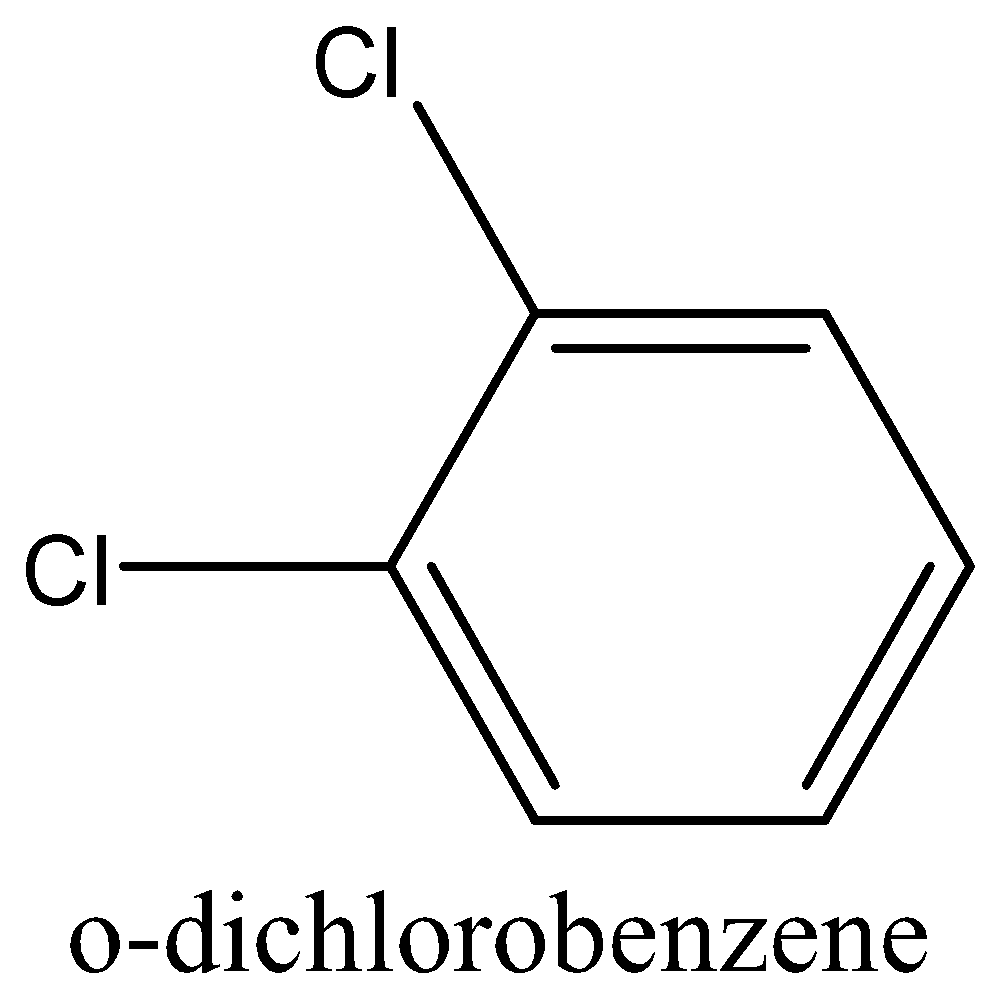
The dipole moment of (IV) p-dichlorobenzene is 0 (zero), (I) toluene is 0.375 D while that of (II) m-dichlorobenzene is 1.48 D and for (III) o-dichlorobenzene is 2.54 D.
The value of dipole moment for each shows that benzene ring is planar and is in\[p - {C_6}{H_4}C{l_2}\], whereas two \[C - Cl\]bonds are directed in the plane of a ring, thus on adding, their result becomes zero.
In the above, two \[C - Cl\] bonds contain an angle of 60° and in \[m - {C_6}{H_4}C{l_2}\] the two \[C - Cl\] bonds contains an angle of 60° and in \[m - {C_6}{H_4}C{l_2}\] the two \[C - Cl\] bonds contains an angle of 120° each and their dipole moments on a vector addition gives a higher value of dipole moment.
In the case of toluene, we can understand that through the methyl group. And the methyl group donates electron density to that carbon and causes a net dipole moment.

In the case of \[o - {C_6}{H_4}C{l_2}\] have higher dipole moments than others dichlorobenzene. This is because of high electronegativity of chlorine placed adjacent to each other in the benzene ring.
Therefore, a sequence of dipole moment is as follows
(IV) P-dichlorobenzene < (I) toluene < (II) m-dichlorobenzene < (III) o-dichlorobenzene
Note: We must know that the dipole moment arises from differences in their electronegativity. We can say that the greater the differences in their electronegativity greater the moment in the dipole for the same. The dipole moment is also a measure of the polarities of the molecule.
Complete step by step answer:
The dipole moment of (IV) p-dichlorobenzene is 0 (zero), (I) toluene is 0.375 D while that of (II) m-dichlorobenzene is 1.48 D and for (III) o-dichlorobenzene is 2.54 D.
The value of dipole moment for each shows that benzene ring is planar and is in\[p - {C_6}{H_4}C{l_2}\], whereas two \[C - Cl\]bonds are directed in the plane of a ring, thus on adding, their result becomes zero.

In the above, two \[C - Cl\] bonds contain an angle of 60° and in \[m - {C_6}{H_4}C{l_2}\] the two \[C - Cl\] bonds contains an angle of 60° and in \[m - {C_6}{H_4}C{l_2}\] the two \[C - Cl\] bonds contains an angle of 120° each and their dipole moments on a vector addition gives a higher value of dipole moment.

In the case of toluene, we can understand that through the methyl group. And the methyl group donates electron density to that carbon and causes a net dipole moment.

In the case of \[o - {C_6}{H_4}C{l_2}\] have higher dipole moments than others dichlorobenzene. This is because of high electronegativity of chlorine placed adjacent to each other in the benzene ring.

Therefore, a sequence of dipole moment is as follows
(IV) P-dichlorobenzene < (I) toluene < (II) m-dichlorobenzene < (III) o-dichlorobenzene
Note: We must know that the dipole moment arises from differences in their electronegativity. We can say that the greater the differences in their electronegativity greater the moment in the dipole for the same. The dipole moment is also a measure of the polarities of the molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




