
The correct increasing bond angle among $B{{F}_{3}}$, $P{{F}_{3}}$ and $Cl{{F}_{3}}$ follows the order:
A.
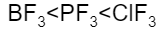
B.
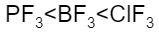
C.
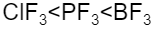
D.
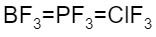
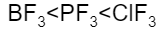
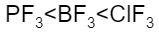
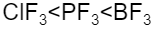
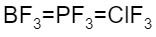
Answer
573.3k+ views
Hint: To solve this question, think about the hybridisation, three-dimensional orientation, and number of lone pairs of electrons on each central atom of the molecule to identify the basic bond angle of the structure and then move on to how lone pairs will affect the bond angle. Then arrange the molecule in increasing order of their bond angle.
Complete step by step solution:
First of all, let us look at the geometry, hybridisation and number of lone pair of electrons of all the molecules given:
- The geometry of $B{{F}_{3}}$ is trigonal planar. It involves four atoms i.e. one boron atom bonded with three fluorine atoms. It has no lone pairs of electrons on its central atom and has a $s{{p}^{2}}$ hybridisation. This means that the bond angle between the atoms will be ${{120}^{o}}$, where all the atoms are in one plane. Each one of them also makes an equilateral triangle. The following figure shows the structure and bond angle of the fluoride:
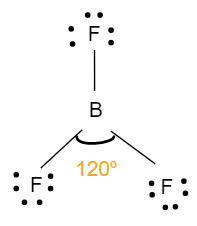
- The geometry of $P{{F}_{3}}$is tetrahedral. It involves four atoms i.e. one phosphorus atom bonded with three fluorine atoms. It has one lone pair of electrons with a hybridisation of $s{{p}^{3}}$.This means that, the bond angle will be less than ${{109}^{o}}$.The structure with bond angle is shown below:
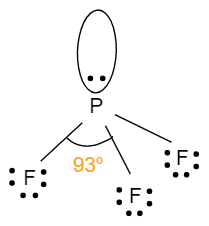
- The geometry of $Cl{{F}_{3}}$ is trigonal bipyramidal. It also involves four atoms in its molecule i.e. one chlorine atom which is bonded with three fluorine atoms. It has two lone pairs of electrons with a hybridisation of $s{{p}^{3}}d$. Due to the presence of lone pairs of electrons, it will have a bond angle less than ${{90}^{o}}$. So, the structure with bond angle is shown below:
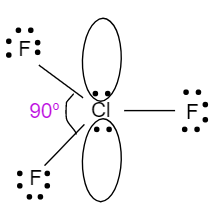
$Cl{{F}_{3}}$ with ${{90}^{o}}$ will be less than $P{{F}_{3}}$ with ${{93}^{o}}$ , which will be less than $B{{F}_{3}}$ with bond angle ${{120}^{o}}$.
Hence, the correct option is C.
Note: Lone pairs are in the orbitals that are shorter and rounder than the orbitals that the bonding pairs occupy. Because of this, there is more repulsion between a lone pair and a bond pair electron thereby forcingly reducing the bond angle. More is the number of lone pairs of electrons, less will be the bond angle of the corresponding molecule.
Complete step by step solution:
First of all, let us look at the geometry, hybridisation and number of lone pair of electrons of all the molecules given:
- The geometry of $B{{F}_{3}}$ is trigonal planar. It involves four atoms i.e. one boron atom bonded with three fluorine atoms. It has no lone pairs of electrons on its central atom and has a $s{{p}^{2}}$ hybridisation. This means that the bond angle between the atoms will be ${{120}^{o}}$, where all the atoms are in one plane. Each one of them also makes an equilateral triangle. The following figure shows the structure and bond angle of the fluoride:

- The geometry of $P{{F}_{3}}$is tetrahedral. It involves four atoms i.e. one phosphorus atom bonded with three fluorine atoms. It has one lone pair of electrons with a hybridisation of $s{{p}^{3}}$.This means that, the bond angle will be less than ${{109}^{o}}$.The structure with bond angle is shown below:

- The geometry of $Cl{{F}_{3}}$ is trigonal bipyramidal. It also involves four atoms in its molecule i.e. one chlorine atom which is bonded with three fluorine atoms. It has two lone pairs of electrons with a hybridisation of $s{{p}^{3}}d$. Due to the presence of lone pairs of electrons, it will have a bond angle less than ${{90}^{o}}$. So, the structure with bond angle is shown below:

$Cl{{F}_{3}}$ with ${{90}^{o}}$ will be less than $P{{F}_{3}}$ with ${{93}^{o}}$ , which will be less than $B{{F}_{3}}$ with bond angle ${{120}^{o}}$.
Hence, the correct option is C.
Note: Lone pairs are in the orbitals that are shorter and rounder than the orbitals that the bonding pairs occupy. Because of this, there is more repulsion between a lone pair and a bond pair electron thereby forcingly reducing the bond angle. More is the number of lone pairs of electrons, less will be the bond angle of the corresponding molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




