
The correct description of the Fischer projection of glyceraldehydes give below, in terms of D & L; R & S and d & l, respectively, is:
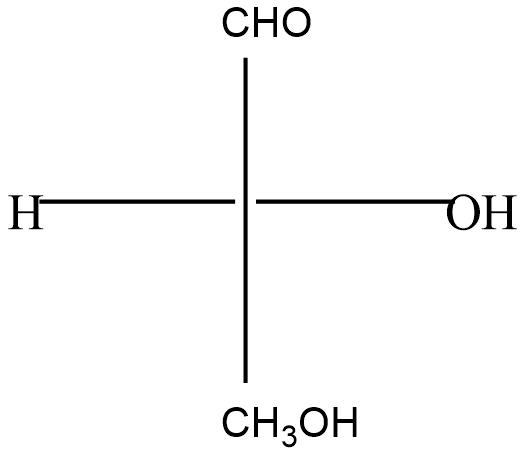
(A) D, R, d
(B) D, R, l
(C) D, S, d
(D) D, S, l

Answer
591k+ views
Hint: The right hand and left-hand nomenclature are used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S. the D-L system corresponds to the configuration of the molecule, spatial arrangement of its atoms around the chirality center. While (+) and (-) notation corresponds to the optical activity of the substance, whether it rotates the plane of polarized light clockwise (+) or counter wise (-).
Complete step by step answer:
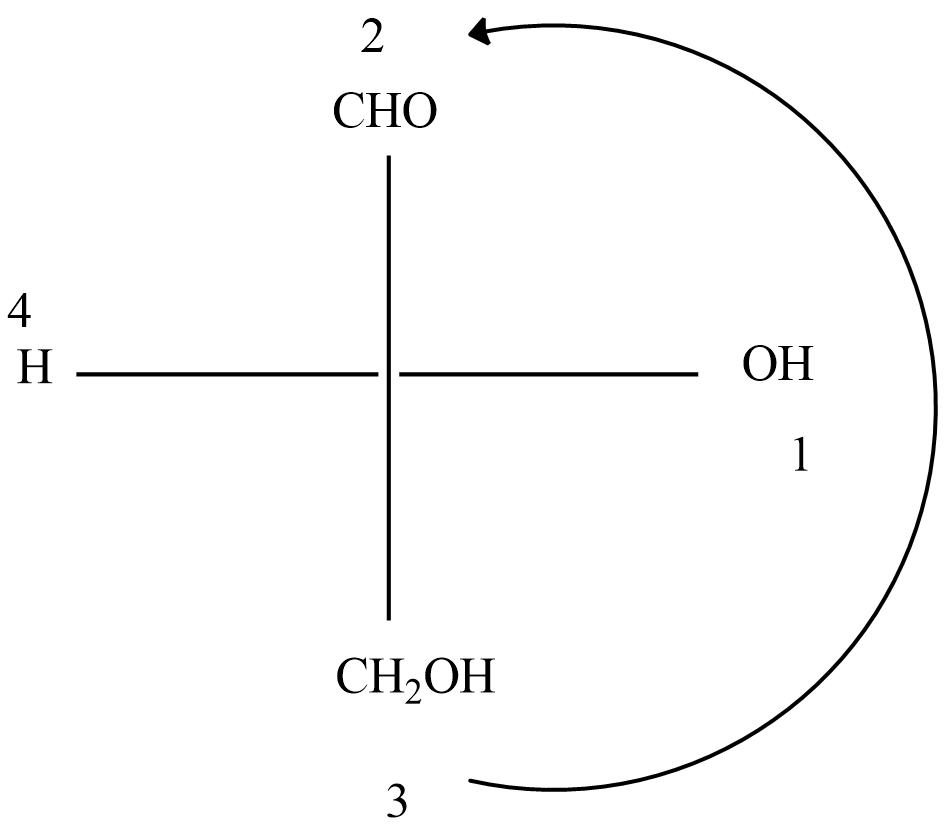
Based on R and S rotation, the above curve arrow shows a clockwise direction based on high priority substituent to low priority substituent which denotes R configuration in glyceraldehyde.
Here, priority numbering given based on the atomic number of the group. Out of four groups, the atomic number of oxygen is high in the -OH group, so it should be given first priority and marked as 1. Similarly after that group, -CHO group gives priority as 2, it goes on priority till least priority for -H atomic number. R isomer similarly equal to (d) + configuration.
Since OH is the right side of which represents D isomer.
So, the correct answer is “Option A”.
Note: A dextrorotatory compound is often prefixed with (+)- or (d)-. Likewise, a levorotatory compound is often prefixed with (-)- or (l)-. These lowercase “d-” or “l-'' prefixes are distinct from the small caps “D-” and “L-” prefixes, which are most often used to distinguish chiral organic compound and are based on the compound’s absolute configuration relative to (+)-glyceraldehyde.
Complete step by step answer:

Based on R and S rotation, the above curve arrow shows a clockwise direction based on high priority substituent to low priority substituent which denotes R configuration in glyceraldehyde.
Here, priority numbering given based on the atomic number of the group. Out of four groups, the atomic number of oxygen is high in the -OH group, so it should be given first priority and marked as 1. Similarly after that group, -CHO group gives priority as 2, it goes on priority till least priority for -H atomic number. R isomer similarly equal to (d) + configuration.
Since OH is the right side of which represents D isomer.
So, the correct answer is “Option A”.
Note: A dextrorotatory compound is often prefixed with (+)- or (d)-. Likewise, a levorotatory compound is often prefixed with (-)- or (l)-. These lowercase “d-” or “l-'' prefixes are distinct from the small caps “D-” and “L-” prefixes, which are most often used to distinguish chiral organic compound and are based on the compound’s absolute configuration relative to (+)-glyceraldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




