
The continued evolution of oxygen in the suspension of an isolated chloroplast in light in the presence of ferric salt, violate dyes, etc. are called-
(a) Oxygenation
(b) Blackmans reaction
(c) Hills reaction
(d) Emerson reaction
Answer
573.9k+ views
Hint: It is formally characterized by the hydrogen of water, with the evolution of oxygen, as the photoreduction of an electron acceptor. The final electron acceptor is NADP+ in vivo, or in the body.
Complete answer:
The Hill reaction is characterized as the photoreduction by the hydrogens of water, with the evolution of oxygen, of an electron acceptor. The final electron acceptor is NADP+ in vivo, or in the body. In isolated chloroplasts, we can calculate the rate of the Hill response. Robert Hill first demonstrated in 1939 that, when incubated in the presence of an artificial electron acceptor (e.g. Ferricyanide) , illuminated chloroplasts produce oxygen. The reaction is a photosystem II property.
Additional information:
Oxygenation: The addition to every system, including the human body, of oxygen. Oxygenation may also refer to the method of treating a patient with oxygen or mixing oxygen with a drug or other material.
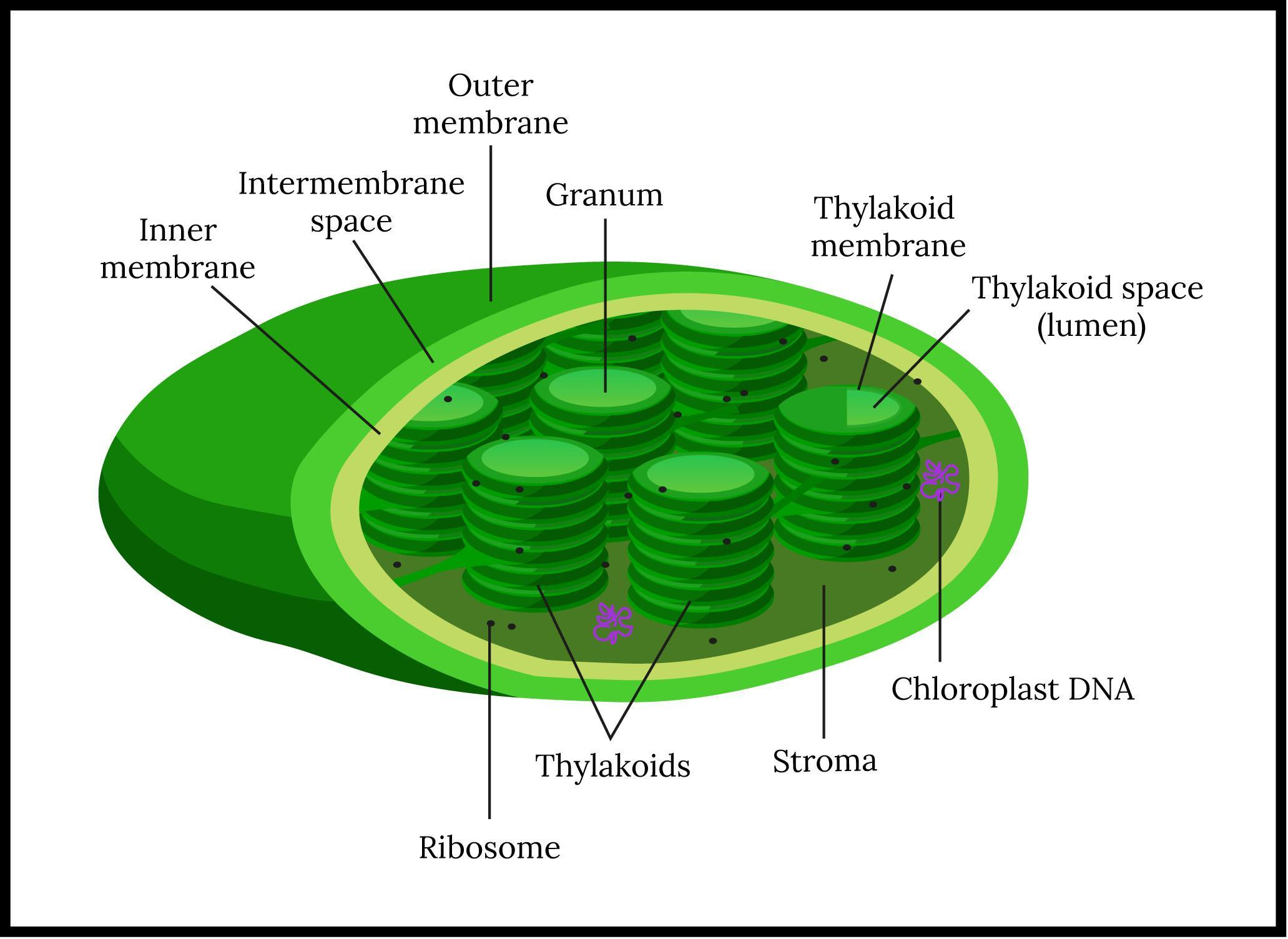
The Emerson effect is an improvement in the rate of photosynthesis after 680 nm (deep red spectrum) and more than 680 nm (far-red spectrum) exposure of chloroplasts to light. The rate of photosynthesis is far higher than the amount of the red light and far-red light photosynthesis rates when exposed to the light of both wavelengths at the same time. The result was early proof that two photosystems collaborate in photosynthesis, processing different wavelengths.
Initially, Blackman clarified the idea of dark reaction in photosynthesis. It is therefore also known by the name of Blackman's answer. This is essentially the step in which the photosynthesis chemical process takes place without the use of sunlight. The reaction occurs in the chloroplast stroma.
So, the correct answer is ‘Hills reaction’.
Note: The Hill reaction is formally characterized as the photoreduction by the hydrogens of water, with the evolution of oxygen, of an electron acceptor. In isolated chloroplasts, we can calculate the rate of the Hill response. As an artificial electron acceptor, this technique uses a dye that changes color as it is reduced.
Complete answer:
The Hill reaction is characterized as the photoreduction by the hydrogens of water, with the evolution of oxygen, of an electron acceptor. The final electron acceptor is NADP+ in vivo, or in the body. In isolated chloroplasts, we can calculate the rate of the Hill response. Robert Hill first demonstrated in 1939 that, when incubated in the presence of an artificial electron acceptor (e.g. Ferricyanide) , illuminated chloroplasts produce oxygen. The reaction is a photosystem II property.
Additional information:
Oxygenation: The addition to every system, including the human body, of oxygen. Oxygenation may also refer to the method of treating a patient with oxygen or mixing oxygen with a drug or other material.

The Emerson effect is an improvement in the rate of photosynthesis after 680 nm (deep red spectrum) and more than 680 nm (far-red spectrum) exposure of chloroplasts to light. The rate of photosynthesis is far higher than the amount of the red light and far-red light photosynthesis rates when exposed to the light of both wavelengths at the same time. The result was early proof that two photosystems collaborate in photosynthesis, processing different wavelengths.
Initially, Blackman clarified the idea of dark reaction in photosynthesis. It is therefore also known by the name of Blackman's answer. This is essentially the step in which the photosynthesis chemical process takes place without the use of sunlight. The reaction occurs in the chloroplast stroma.
So, the correct answer is ‘Hills reaction’.
Note: The Hill reaction is formally characterized as the photoreduction by the hydrogens of water, with the evolution of oxygen, of an electron acceptor. In isolated chloroplasts, we can calculate the rate of the Hill response. As an artificial electron acceptor, this technique uses a dye that changes color as it is reduced.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




