
The compound B in below given reaction is-
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CN + C}}{{\text{H}}_{\text{3}}}{\text{MgI}} \to {\text{A}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O/H + }}}}{\text{B}}\]
A) Acetic acid
B) Acetone
C) Acetaldehyde
D) Ethyl alcohol
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CN}}\] is known as acetonitrile hamada.
Answer
526.5k+ views
Hint: We have to remember that the Grignard reagent is one of the organometallic compounds. Organometallic compound means that compound has a carbon metal direct bond. In Grignard reagent all are having a divalent metal. Grignard reagent almost having in magnesium metal only. The bond between carbon atom and magnesium metal is normally covalent only but highly polar. In organic chemistry, carbon are mostly less electronegativity, but compare with metal it having more electronegativity. That the reason here carbon having partial negative charge and magnesium having partial positive charge.
Complete answer:
The given reaction is,
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CN + C}}{{\text{H}}_{\text{3}}}{\text{MgI}} \to {\text{A}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O/H + }}}}{\text{B}}\]
The acetonitrile is reacted with methyl magnesium iodine to give intermediate of some imide metallic compound. Further we go for hydrolysis, lone pairs of electrons in oxygen going to bond in carbon atoms and form acetone as the main product of the Grignard reaction.
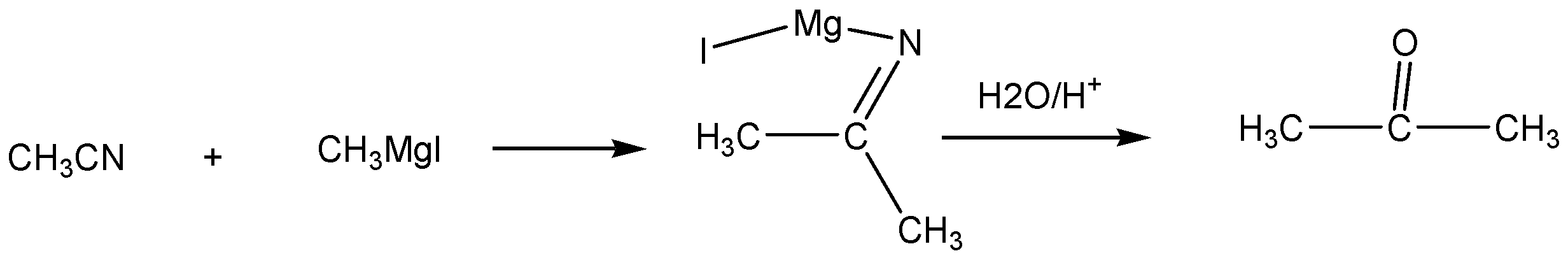
The compound is acetone. The functional group is acetone and ketone.
Option A is not correct, because acetic acid is not compound B.
Option C is not correct, because acetaldehyde is not compound B.
Option is not correct, because ethyl alcohol is not compound B.
Option B is correct, because acetone is compound B.
Note:
We have to know that the general representation of Grignard reagent is \[{\text{RMgX}}\]. Here R is represent as the alkaline group. It is organic nature in organometallic compound. X is represent as the halogen group. In Grignard mostly used as chlorine, bromine and iodine. Florine is not suitable for Grignard reaction because of the size. Depend on the product and reaction condition we choose \[{\text{RMgCl}}\], \[{\text{RMgBr}}\] and \[{\text{RMgI}}\].
Complete answer:
The given reaction is,
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CN + C}}{{\text{H}}_{\text{3}}}{\text{MgI}} \to {\text{A}}\xrightarrow{{{{\text{H}}_{\text{2}}}{\text{O/H + }}}}{\text{B}}\]
The acetonitrile is reacted with methyl magnesium iodine to give intermediate of some imide metallic compound. Further we go for hydrolysis, lone pairs of electrons in oxygen going to bond in carbon atoms and form acetone as the main product of the Grignard reaction.

The compound is acetone. The functional group is acetone and ketone.
Option A is not correct, because acetic acid is not compound B.
Option C is not correct, because acetaldehyde is not compound B.
Option is not correct, because ethyl alcohol is not compound B.
Option B is correct, because acetone is compound B.
Note:
We have to know that the general representation of Grignard reagent is \[{\text{RMgX}}\]. Here R is represent as the alkaline group. It is organic nature in organometallic compound. X is represent as the halogen group. In Grignard mostly used as chlorine, bromine and iodine. Florine is not suitable for Grignard reaction because of the size. Depend on the product and reaction condition we choose \[{\text{RMgCl}}\], \[{\text{RMgBr}}\] and \[{\text{RMgI}}\].
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




