
The $ {C_6}{H_5}C{H_3}\xrightarrow{{Oxidation}}A\xrightarrow{{NaOH}}B\xrightarrow{{{\text{Soda lime, }}\Delta }}C $
Identify the product C in the above given reaction sequence.
(A) $ {C_6}{H_5}OH $
(B) $ {C_6}{H_6} $
(C) $ {C_6}{H_5}COONa $
(D) $ {C_6}{H_5}ONa $
Answer
548.4k+ views
Hint: Oxidation reaction depends upon the reactants undergoing oxidation. $ {C_6}{H_5}C{H_3} $ is also known as toluene. Oxidation results in formation of carboxylic acid which neutralizes salt with NaOH and the third reaction is soda lime decarboxylation reaction.
Complete Step by step solution
The reactant in the reaction is toluene. Toluene undergoes oxidation to form benzoic acid. Oxidation can take place here due to the reagent potassium permanganate in acidic condition. Partial oxidation of toluene gives benzaldehyde and complete oxidation of toluene gives benzoic acid. Other than potassium permanganate, acidic potassium dichromate or nitric acid can also be used to convert toluene into benzoic acid.
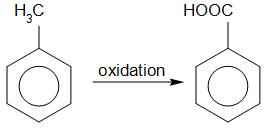
Toluene Benzoic acid
Hence the compound A is benzoic acid.
Here the reactant is benzoic acid. Benzoic acid reacts with aqueous sodium hydroxide to form a salt of sodium benzoate. Sodium benzoate formed dissolves in an aqueous solution. This tells us that sodium benzoate is soluble in water due to the presence of ions in it.
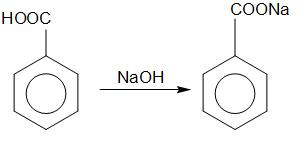
Benzoic acid Sodium benzoate
Hence the compound B is sodium benzoate.
Here, the reactant is sodium benzoate. Sodium benzoate is used to preserve beverages. The reaction of sodium benzoate with soda lime involves decarboxylation reaction. In a decarboxylation reaction, carbon dioxide is removed from a compound. Here the carbon dioxide removed combines to form sodium carbonate. Sodium benzoate after undergoing decarboxylation forms benzene as a product.
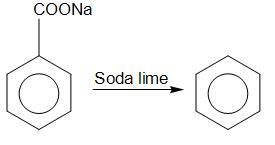
Sodium benzoate Benzene
Hence the compound C is benzene. The molecular formula of benzene is $ {C_6}{H_6} $ .
Therefore, the correct answer is option B.
Note
Benzene is used as an intermediate for a number of reactions. Benzene increases the octane rating. Benzene is regarded as the basic structure for any aromatic compound.
Complete Step by step solution
The reactant in the reaction is toluene. Toluene undergoes oxidation to form benzoic acid. Oxidation can take place here due to the reagent potassium permanganate in acidic condition. Partial oxidation of toluene gives benzaldehyde and complete oxidation of toluene gives benzoic acid. Other than potassium permanganate, acidic potassium dichromate or nitric acid can also be used to convert toluene into benzoic acid.

Toluene Benzoic acid
Hence the compound A is benzoic acid.
Here the reactant is benzoic acid. Benzoic acid reacts with aqueous sodium hydroxide to form a salt of sodium benzoate. Sodium benzoate formed dissolves in an aqueous solution. This tells us that sodium benzoate is soluble in water due to the presence of ions in it.

Benzoic acid Sodium benzoate
Hence the compound B is sodium benzoate.
Here, the reactant is sodium benzoate. Sodium benzoate is used to preserve beverages. The reaction of sodium benzoate with soda lime involves decarboxylation reaction. In a decarboxylation reaction, carbon dioxide is removed from a compound. Here the carbon dioxide removed combines to form sodium carbonate. Sodium benzoate after undergoing decarboxylation forms benzene as a product.

Sodium benzoate Benzene
Hence the compound C is benzene. The molecular formula of benzene is $ {C_6}{H_6} $ .
Therefore, the correct answer is option B.
Note
Benzene is used as an intermediate for a number of reactions. Benzene increases the octane rating. Benzene is regarded as the basic structure for any aromatic compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




