
The chemical formula of the king of chemicals, Sulphuric acid is:
A.$H_{ 2 }S_{ 2 }O_{ 3 }$
B.$H_{ 2 }SO_{ 3 }$
C.$HSO_{ 4 }$
D.$H_{ 2 }SO_{ 4 }$
Answer
593.1k+ views
Hint: The acid which is known as “the king of acids” is a strong acid. This is also the most widely used chemical. Now, you try to identify the strongest acid among all the options provided.
Complete step by step answer:
Let’s look at all the options one by one -
-Option A, $H_{ 2 }S_{ 2 }O_{ 3 }$ is ThioSulphuric acid that is a sulfur oxoacid. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water.
-Option B, $H_{ 2 }SO_{ 3 }$ is Sulfurous acid. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. The conjugate bases of this elusive acid are common anions, bisulfite, and sulfite.
-Option C, $HSO_{ 4 }$ this chemical formula doesn’t exist.
-Option D is Sulphuric acid, which is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with molecular formula $H_{ 2 }SO_{ 4 }$. It is called the king of acid because of its direct and indirect applications in the manufacture of many chemicals including fertilizers. So we can say, it is ideal to call sulphuric acid as the king of chemicals.
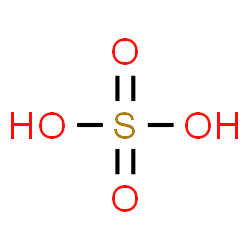
Some chemical properties of sulphuric acid:
-Oily liquid
-Soluble in water (and will release heat)
-Corrosive to tissue and metals
-Very harmful and can cause serious health effects if inhaled or ingested
Therefore,the correct option is D.
Note:
In option C, if it is $HSO_{ 4 }^{ - }$ (same species with -1 charge), then it is known as ‘hydrogen sulfate ion’ or ‘bisulfate ion’. Bisulfates are usually acid salts, created when sulphuric acid meets a metallic base.
Complete step by step answer:
Let’s look at all the options one by one -
-Option A, $H_{ 2 }S_{ 2 }O_{ 3 }$ is ThioSulphuric acid that is a sulfur oxoacid. The acid cannot be made by acidifying aqueous thiosulfate salt solutions as the acid readily decomposes in water.
-Option B, $H_{ 2 }SO_{ 3 }$ is Sulfurous acid. There is no evidence that sulfurous acid exists in solution, but the molecule has been detected in the gas phase. The conjugate bases of this elusive acid are common anions, bisulfite, and sulfite.
-Option C, $HSO_{ 4 }$ this chemical formula doesn’t exist.
-Option D is Sulphuric acid, which is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with molecular formula $H_{ 2 }SO_{ 4 }$. It is called the king of acid because of its direct and indirect applications in the manufacture of many chemicals including fertilizers. So we can say, it is ideal to call sulphuric acid as the king of chemicals.

Some chemical properties of sulphuric acid:
-Oily liquid
-Soluble in water (and will release heat)
-Corrosive to tissue and metals
-Very harmful and can cause serious health effects if inhaled or ingested
Therefore,the correct option is D.
Note:
In option C, if it is $HSO_{ 4 }^{ - }$ (same species with -1 charge), then it is known as ‘hydrogen sulfate ion’ or ‘bisulfate ion’. Bisulfates are usually acid salts, created when sulphuric acid meets a metallic base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




