
The canonical or resonating structures of a molecule required to describe the structure of a molecule follow which of the following rules?
A.The relative position of all atoms can differ
B.The same number of unpaired and paired electrons in all structures
C.The energy of each structure is different
D.Like charges are present on adjacent atoms
Answer
573.6k+ views
Hint: To answer this question recall the properties of resonance structures. Resonance structures of any molecule are in which the chemical interaction and properties remain the same, but the electrons are distributed around the structure differently.
Complete step by step answer:
We know from the theory of resonating structures that they are the multiple structures with similar energy, the position of nuclei, bonding and the nonbonding pair of electrons. They are known as canonical structures and the molecule exists in nature as a superimposition of multiple Lewis structures. Let’s see an example of resonating structures:
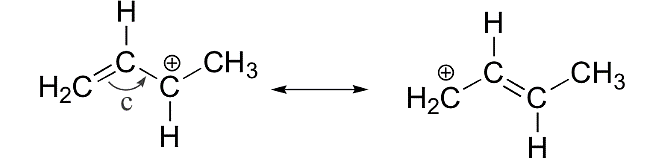
An example of resonating structures
-From the concept of resonating structures, we can say the following about existence of resonating structures of a molecule:
-Same positions of all atoms.
-The same number of valence electrons as electrons are not created or destroyed.
Equivalent energy.
-Opposite charges on adjacent atoms to regain stability. If like charges are present on adjacent atoms, the resonating structure will be highly unstable.
Hence option B is correct.
Note:
There are two components to evaluating resonance structures - whether it is "legal" and whether it is "significant". The key to drawing resonance structures is to make sure you are not breaking or creating any new single bonds. The connectivity between atoms should stay the same. As long as you stick to that, the structures you draw should be valid resonance structures. Determining which structures are energetically reasonable is something that requires a bit more practice.
Complete step by step answer:
We know from the theory of resonating structures that they are the multiple structures with similar energy, the position of nuclei, bonding and the nonbonding pair of electrons. They are known as canonical structures and the molecule exists in nature as a superimposition of multiple Lewis structures. Let’s see an example of resonating structures:

An example of resonating structures
-From the concept of resonating structures, we can say the following about existence of resonating structures of a molecule:
-Same positions of all atoms.
-The same number of valence electrons as electrons are not created or destroyed.
Equivalent energy.
-Opposite charges on adjacent atoms to regain stability. If like charges are present on adjacent atoms, the resonating structure will be highly unstable.
Hence option B is correct.
Note:
There are two components to evaluating resonance structures - whether it is "legal" and whether it is "significant". The key to drawing resonance structures is to make sure you are not breaking or creating any new single bonds. The connectivity between atoms should stay the same. As long as you stick to that, the structures you draw should be valid resonance structures. Determining which structures are energetically reasonable is something that requires a bit more practice.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




