
The ${\text{C}} - {\text{O}} - {\text{C}}$ bond angle in dimethyl ether is:
A. ${111.7^ \circ }$
B. ${108.5^ \circ }$
C. ${109^ \circ }$
D. ${120^ \circ }$
Answer
569.7k+ views
Hint: Ether is a compound having alkyl groups which is connected with an oxygen atom. Each carbon and one oxygen is ${\text{s}}{{\text{p}}^3}$ hybridized. Dimethyl ether has a bent structure. It is also known as methoxy methane since two methyl groups are attached.
Complete step by step solution:
The structure of dimethyl ether is given below:
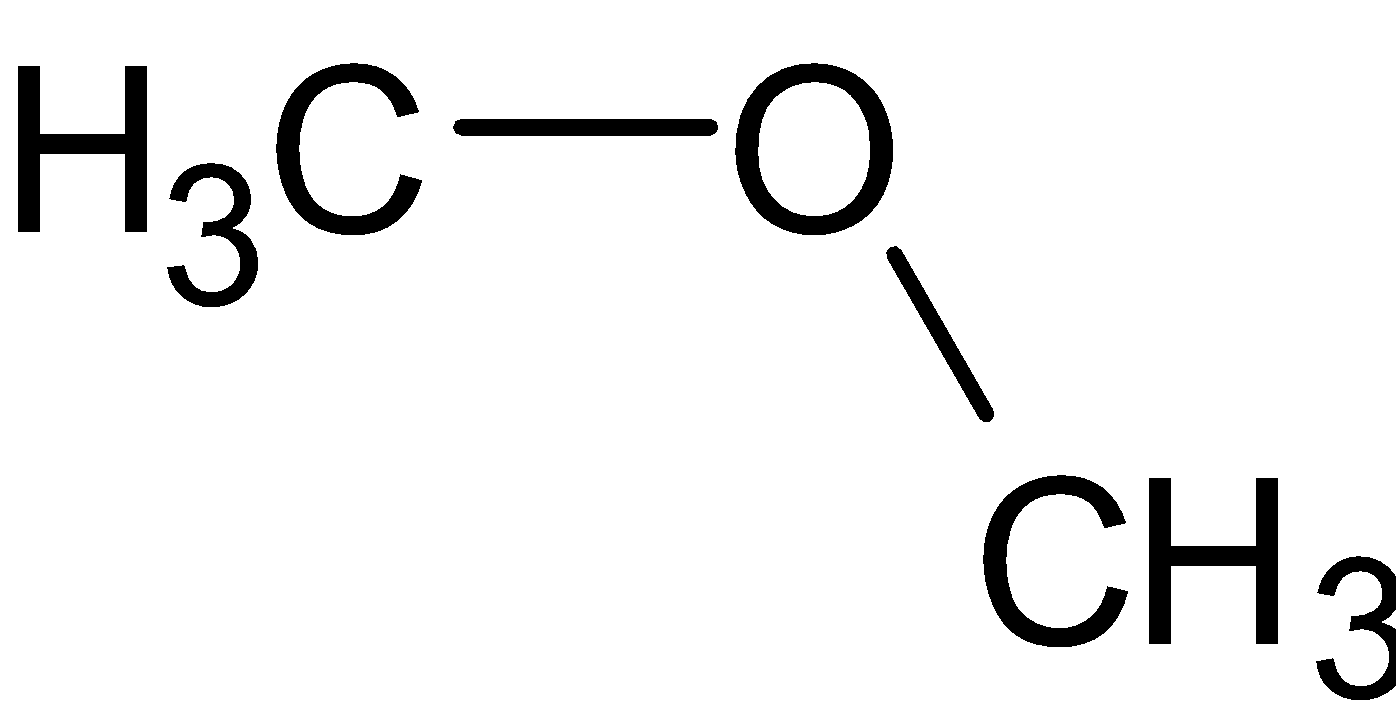
This is a bent structure. But it is unlike the structure of water molecules. Both have different bond angles.
Dimethyl ether is slightly polar. The oxygen atom in the middle has bonded to two carbon atoms. Still it has two lone pairs of electrons. We know that carbon and oxygen atoms are highly electronegative. But their electronegativity difference is very small since they lie near to each other in a periodic table.
To a first approximation, we assume that the bond angle is ${109.5^ \circ }$ as we have seen in water molecules. But this is not the case. In this molecule, the two lone pairs of electrons in the oxygen tries to lie closer to the oxygen atom. But since the side groups are heavy, the repulsion of lone pairs of electrons cannot compress them. Thus the bond angle will be ${111.7^ \circ }$.
Hence, the correct option is A.
Note: The bond angle of ${\text{C}} - {\text{O}} - {\text{C}}$ in dimethyl ether is greater than the bond angle of ${\text{H - O - H}}$ in water. The methyl group is heavier than the hydrogen atom since both act as side groups. Thus the effect of lone-pair electron repulsion is more in water molecules because since the methyl group is heavier, the repulsion will not be that much effective. Thus it has more than the bond angle of ${\text{H - O - H}}$ in water, i.e. ${109.5^ \circ }$.
Complete step by step solution:
The structure of dimethyl ether is given below:

This is a bent structure. But it is unlike the structure of water molecules. Both have different bond angles.
Dimethyl ether is slightly polar. The oxygen atom in the middle has bonded to two carbon atoms. Still it has two lone pairs of electrons. We know that carbon and oxygen atoms are highly electronegative. But their electronegativity difference is very small since they lie near to each other in a periodic table.
To a first approximation, we assume that the bond angle is ${109.5^ \circ }$ as we have seen in water molecules. But this is not the case. In this molecule, the two lone pairs of electrons in the oxygen tries to lie closer to the oxygen atom. But since the side groups are heavy, the repulsion of lone pairs of electrons cannot compress them. Thus the bond angle will be ${111.7^ \circ }$.
Hence, the correct option is A.
Note: The bond angle of ${\text{C}} - {\text{O}} - {\text{C}}$ in dimethyl ether is greater than the bond angle of ${\text{H - O - H}}$ in water. The methyl group is heavier than the hydrogen atom since both act as side groups. Thus the effect of lone-pair electron repulsion is more in water molecules because since the methyl group is heavier, the repulsion will not be that much effective. Thus it has more than the bond angle of ${\text{H - O - H}}$ in water, i.e. ${109.5^ \circ }$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




