
What would be the basicity of pyro-phosphoric acid?
A. $4$
B. $3$
C. $2$
D. $5$
Answer
541.5k+ views
Hint: As we know that basicity of an acid is basically the number of hydronium ions given by an acid during a course of reaction or in general. We also know that pyro-phosphoric acid is the glassy solid organic compound which is obtained by the heating of phosphoric acid.
Complete step by step answer:
As we have learnt that pyro-phosphoric acid is the glassy solid organic compound which is obtained by the heating of phosphoric acid resulting in the chemical formula as ${H_4}{P_2}{O_7}$.
We also know that basicity of an acid is basically defined as the number of hydronium ions given by an acid during a course of reaction or in general.
We can show this with the help of the structure of pyro-phosphoric acid as given below:
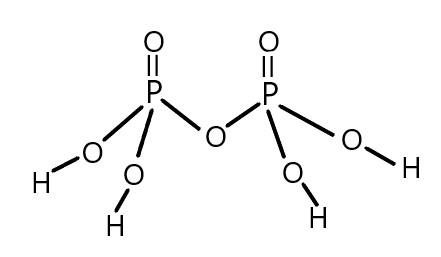
So, we can conclude that pyro-phosphoric acid having four hydronium ions can be easily released by this acid so we can say that the basicity of pyro-phosphoric acid is $4$ .
So, the correct answer is Option A.
Additional information:
one of the best methods of preparation of pyro-phosphoric acid is by ion exchange from sodium pyrophosphate or by the treatment of lead pyrophosphate and hydrogen sulphide. Pyro-phosphoric acid is corrosive in nature but it is non-toxic. Pyro-phosphoric acid was accidentally discovered by Clark by heating the sodium phosphate salt to red heat and when phosphoric acid was heated to red heat it resulted into the formation of pyro-phosphoric acid.
Note: At equilibrium, pyro-phosphoric acid in molten state forms a mixture of phosphoric acid, pyro-phosphoric acid and poly-phosphoric acid. Just like the basicity of an acid we can calculate the acidity of a base by counting the number of replaceable hydroxide ions.
Complete step by step answer:
As we have learnt that pyro-phosphoric acid is the glassy solid organic compound which is obtained by the heating of phosphoric acid resulting in the chemical formula as ${H_4}{P_2}{O_7}$.
We also know that basicity of an acid is basically defined as the number of hydronium ions given by an acid during a course of reaction or in general.
We can show this with the help of the structure of pyro-phosphoric acid as given below:

So, we can conclude that pyro-phosphoric acid having four hydronium ions can be easily released by this acid so we can say that the basicity of pyro-phosphoric acid is $4$ .
So, the correct answer is Option A.
Additional information:
one of the best methods of preparation of pyro-phosphoric acid is by ion exchange from sodium pyrophosphate or by the treatment of lead pyrophosphate and hydrogen sulphide. Pyro-phosphoric acid is corrosive in nature but it is non-toxic. Pyro-phosphoric acid was accidentally discovered by Clark by heating the sodium phosphate salt to red heat and when phosphoric acid was heated to red heat it resulted into the formation of pyro-phosphoric acid.
Note: At equilibrium, pyro-phosphoric acid in molten state forms a mixture of phosphoric acid, pyro-phosphoric acid and poly-phosphoric acid. Just like the basicity of an acid we can calculate the acidity of a base by counting the number of replaceable hydroxide ions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




