
The alkyl halide is converted into alcohol by:
a.) Elimination
b.) Halogenation
c.) Addition
d.) Substitution
Answer
600k+ views
Hint: Alkyl halides also known as haloalkanes. Haloalkanes are the compounds in which one or more hydrogen atoms in an alkane have been replaced with halogen atoms (fluorine, chlorine, bromine etc.). The halides can be exchanged with hydroxyl groups by using a specific type of reaction.
Complete answer:
The alkyl halides are going to be represented with R-X. (R= alkanes, X= halides or halogens.)
Now in the question it is mentioned that alkyl halide is converted into alcohol.
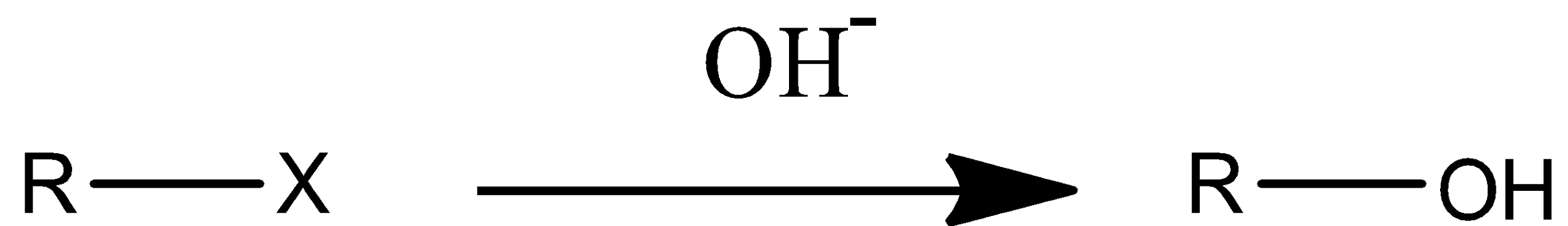
From the above reaction we can understand very clearly that by substitution reaction only we can prepare alcohols from alkyl halides.
So, the correct option is D.
Additional information
There are two types of substitution reactions, \[S{{N}^{1}}\] and \[S{{N}^{2}}\].
\[S{{N}^{1}}\]-Unimolecular substitution reaction.
\[S{{N}^{2}}\]- Bimolecular substitution reaction.
Primary and secondary alkyl halides undergo \[S{{N}^{2}}\]reactions in presence of base to form alcohols.
Tertiary alkyl halide undergoes\[S{{N}^{1}}\] reactions in presence of base to form alcohols.
\[S{{N}^{1}}\] is a single step reaction and \[S{{N}^{2}}\] is a two step reaction.
\[S{{N}^{1}}\] is a fast reaction and \[S{{N}^{2}}\] is a slow reaction.
In \[S{{N}^{1}}\]racemic mixture is going to form and in \[S{{N}^{2}}\] only a single product is going to form.
Note: Don’t be confused with the terms addition reaction, elimination reaction and substitution reactions.
Additional reactions: this type of reactions are possible in unsaturated compounds (Compounds having double or triple bonds.
Elimination reactions: An elimination reaction in which two substituents are removed from a molecule in either one or two-step mechanism and forms unsaturated products at the end of the reaction.
Substitution reactions: Substitution reaction is a chemical reaction during which one functional group in a molecule can be replaced by another functional group.
Complete answer:
The alkyl halides are going to be represented with R-X. (R= alkanes, X= halides or halogens.)
Now in the question it is mentioned that alkyl halide is converted into alcohol.
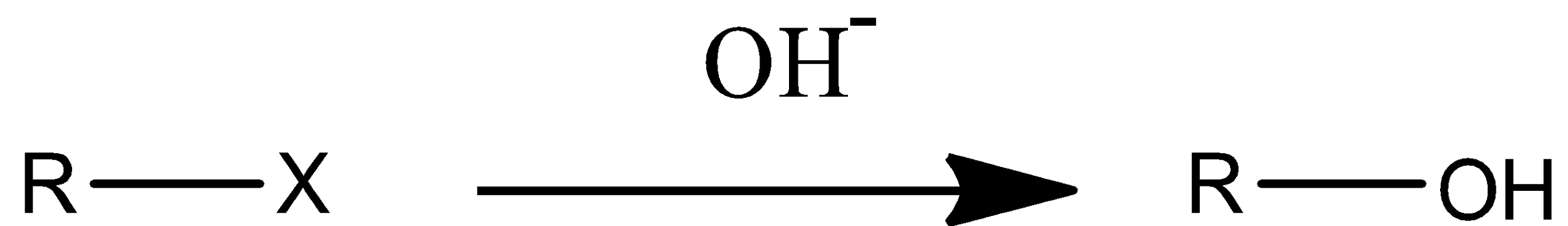
From the above reaction we can understand very clearly that by substitution reaction only we can prepare alcohols from alkyl halides.
So, the correct option is D.
Additional information
There are two types of substitution reactions, \[S{{N}^{1}}\] and \[S{{N}^{2}}\].
\[S{{N}^{1}}\]-Unimolecular substitution reaction.
\[S{{N}^{2}}\]- Bimolecular substitution reaction.
Primary and secondary alkyl halides undergo \[S{{N}^{2}}\]reactions in presence of base to form alcohols.
Tertiary alkyl halide undergoes\[S{{N}^{1}}\] reactions in presence of base to form alcohols.
\[S{{N}^{1}}\] is a single step reaction and \[S{{N}^{2}}\] is a two step reaction.
\[S{{N}^{1}}\] is a fast reaction and \[S{{N}^{2}}\] is a slow reaction.
In \[S{{N}^{1}}\]racemic mixture is going to form and in \[S{{N}^{2}}\] only a single product is going to form.
Note: Don’t be confused with the terms addition reaction, elimination reaction and substitution reactions.
Additional reactions: this type of reactions are possible in unsaturated compounds (Compounds having double or triple bonds.
Elimination reactions: An elimination reaction in which two substituents are removed from a molecule in either one or two-step mechanism and forms unsaturated products at the end of the reaction.
Substitution reactions: Substitution reaction is a chemical reaction during which one functional group in a molecule can be replaced by another functional group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




