
The alkene which on ozonolysis yields acetone is
A.
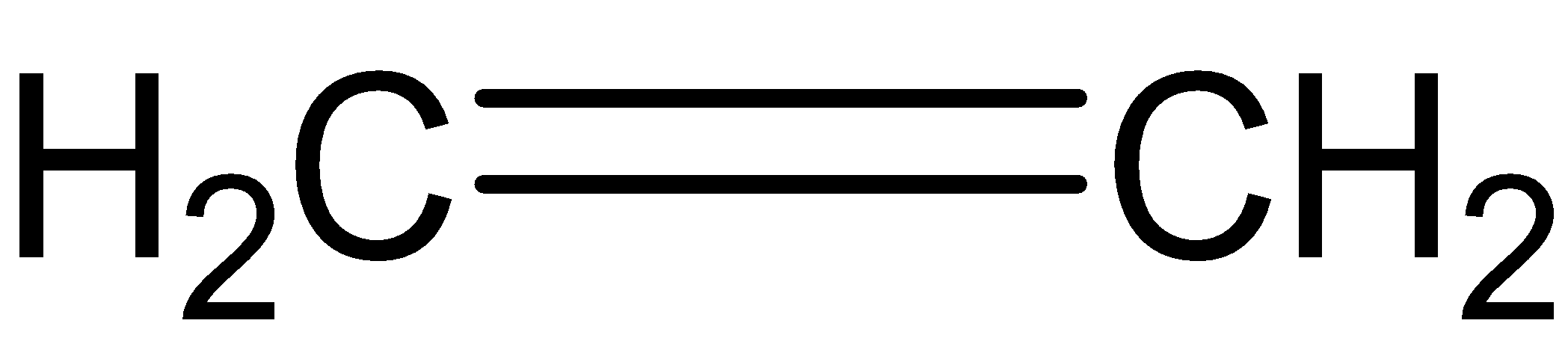
B.
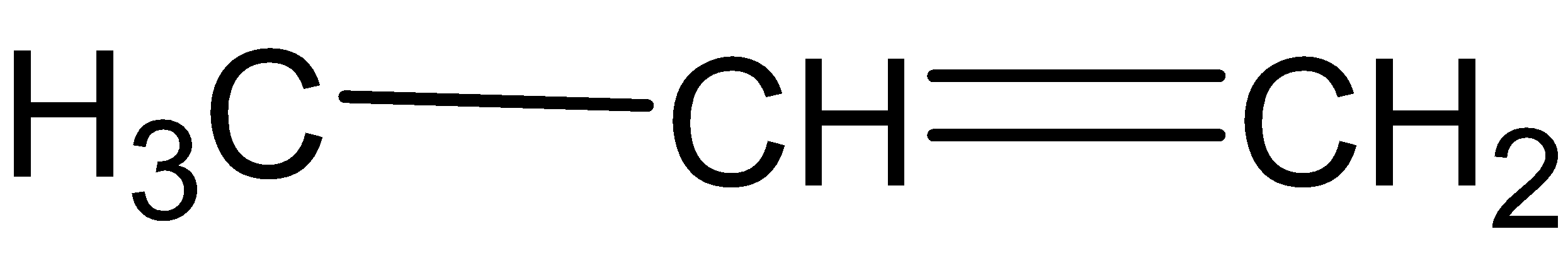
C.
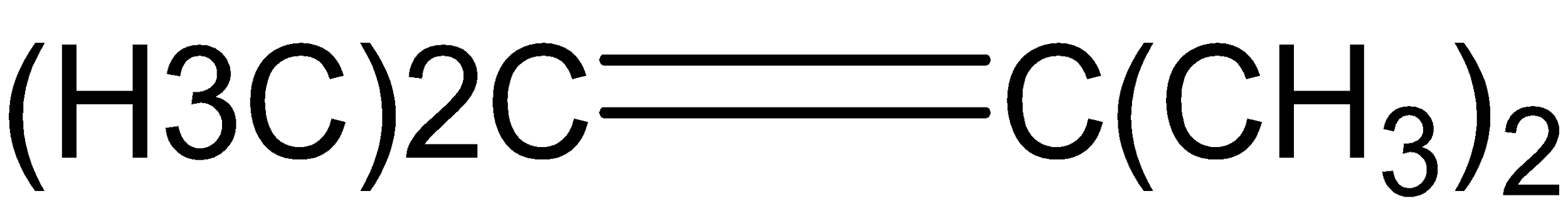
D.
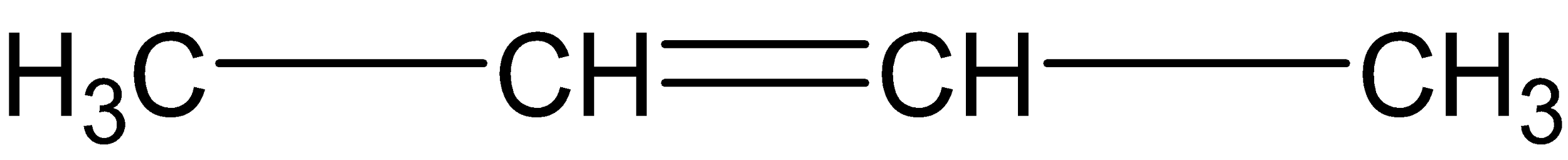
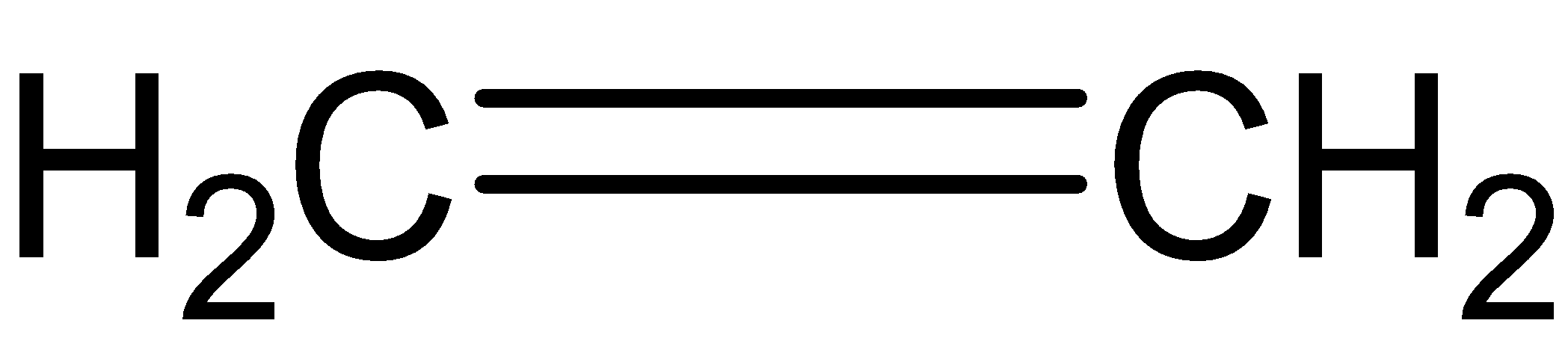

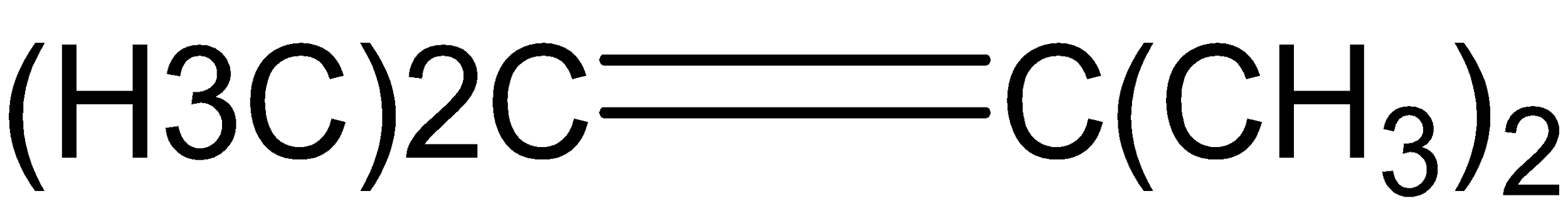
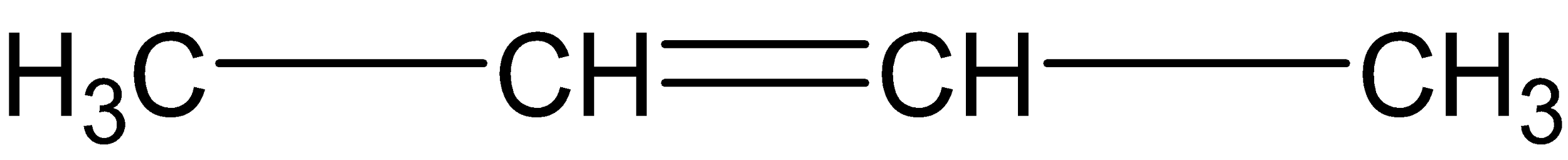
Answer
361.8k+ views
Hint: The ozonolysis of alkenes is a procedure of oxidatively cleaving alkenes or alkynes using ozone, ${{O}_{3}}$. By this method, carbon-carbon double bonds or triple bonds are replaced by double bonds with oxygen. In this problem, we will have to leave the double bonds of the given alkene and replace them with double bonds with oxygen to get the desired acetone.
Complete Step by Step Answer:
Ozonolysis is a method in which ozone cleaves the unsaturated bonds of alkenes, alkynes, or azo compounds. Thereby cleaving the double bonded oxygen alkene and alkynes produce carbonyl compounds whereas azo compounds produce nitrosamines. Through this oxidizing process alcohols, ketones, aldehydes as well as carboxylic acids can be formed.
The intermediate ozonide is further converted to a carbonyl derivative after completion of the workup. This work may be reductive or oxidative. Carboxylic acids are formed by oxidative work up and aldehydes or ketones are formed by a reductive workup.
If ozonolysis occurs of compound (A), we get two molecules of formaldehyde.
When ozonolysis occurs in (B) we get one molecule of formaldehyde and one molecule of acetaldehyde.
Ozone cleaves the double bond of compound (C), and only then produces two molecules of acetone.
Finally ozonolysis in alkene (D), acetones are not formed. Two molecules of acetaldehyde are formed here.
Therefore, alkene (C) produces acetone on ozonolysis.
Thus, option (C) is correct.
Note: In ozonolysis if we use hydrogen peroxide for oxidative workup instead of zinc, dimethyl ether, etc, aldehydes are further oxidized to carboxylic acid. If in the reaction medium zinc of dimethyl ether is present, aldehydes or ketones are formed through a reductive workup.
Complete Step by Step Answer:
Ozonolysis is a method in which ozone cleaves the unsaturated bonds of alkenes, alkynes, or azo compounds. Thereby cleaving the double bonded oxygen alkene and alkynes produce carbonyl compounds whereas azo compounds produce nitrosamines. Through this oxidizing process alcohols, ketones, aldehydes as well as carboxylic acids can be formed.
The intermediate ozonide is further converted to a carbonyl derivative after completion of the workup. This work may be reductive or oxidative. Carboxylic acids are formed by oxidative work up and aldehydes or ketones are formed by a reductive workup.
If ozonolysis occurs of compound (A), we get two molecules of formaldehyde.
When ozonolysis occurs in (B) we get one molecule of formaldehyde and one molecule of acetaldehyde.
Ozone cleaves the double bond of compound (C), and only then produces two molecules of acetone.
Finally ozonolysis in alkene (D), acetones are not formed. Two molecules of acetaldehyde are formed here.
Therefore, alkene (C) produces acetone on ozonolysis.
Thus, option (C) is correct.
Note: In ozonolysis if we use hydrogen peroxide for oxidative workup instead of zinc, dimethyl ether, etc, aldehydes are further oxidized to carboxylic acid. If in the reaction medium zinc of dimethyl ether is present, aldehydes or ketones are formed through a reductive workup.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Difference Between Plant Cell and Animal Cell




