
The alkane that yields two isomeric monobromo derivatives is
A.Neopentane
B.Ethane
C.Methane
D.Propane
Answer
500.4k+ views
Hint: Alkanes are unsaturated hydrocarbons. Due to the absence of much electronegativity difference between the atoms in alkanes. These compounds undergo only substitution reactions like reactions with bromine. Propane is an alkane react with bromine to form two monobromo derivatives.
Complete answer:
Hydrocarbons are the chemical compounds that consist of only carbon and hydrogen atoms. Alkanes are saturated hydrocarbons as they do not have any unsaturation like double or triple bonds.
Carbon and hydrogen do not have much difference in the value of electronegativity. Thus, there is no net displacement of charge between these two atoms Which leads to the inertness of alkanes.
Neopentane is an alkane with five carbon atoms and forms a monobromo derivative.
Ethane is an alkane with two carbon atoms and forms a monobromo derivative.
Methane is an alkane with one carbon atom and forms a monobromo derivative.
Propane is an alkane with three carbon atoms and forms two monobromo derivatives. Thus, the alkane that yields two isomeric monobromo derivatives is propane.
The formation of two monobromo derivatives from propane will be as follows:
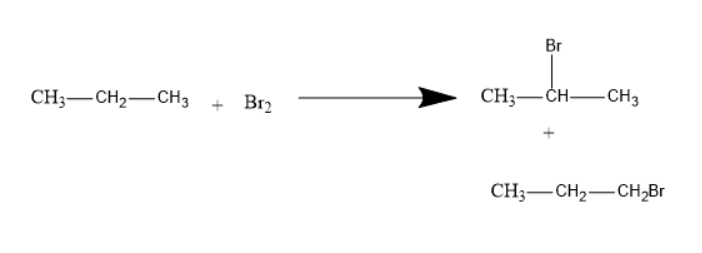
Option D is the correct one.
Note:
Alkanes react with bromine to form bromo alkanes. Bromine is a halogen, but bromine gas has one electrophilic bromine and the other is nucleophilic bromine. Electrophilic bromine will be substituted in the place of hydrogen in alkanes. As propane is unsymmetrical it forms two monobromo derivatives.
Complete answer:
Hydrocarbons are the chemical compounds that consist of only carbon and hydrogen atoms. Alkanes are saturated hydrocarbons as they do not have any unsaturation like double or triple bonds.
Carbon and hydrogen do not have much difference in the value of electronegativity. Thus, there is no net displacement of charge between these two atoms Which leads to the inertness of alkanes.
Neopentane is an alkane with five carbon atoms and forms a monobromo derivative.
Ethane is an alkane with two carbon atoms and forms a monobromo derivative.
Methane is an alkane with one carbon atom and forms a monobromo derivative.
Propane is an alkane with three carbon atoms and forms two monobromo derivatives. Thus, the alkane that yields two isomeric monobromo derivatives is propane.
The formation of two monobromo derivatives from propane will be as follows:

Option D is the correct one.
Note:
Alkanes react with bromine to form bromo alkanes. Bromine is a halogen, but bromine gas has one electrophilic bromine and the other is nucleophilic bromine. Electrophilic bromine will be substituted in the place of hydrogen in alkanes. As propane is unsymmetrical it forms two monobromo derivatives.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




