
Terylene is a condensation polymer of Ethylene glycol and ____________.
A) Salicylic acid
B) Phthalic acid
C) Benzoic acid
D) Terephthalic acid
Answer
592.2k+ views
Hint: The polymer used in this polymerisation condensation process is easily available and easy to do esterification with the polymer and in this process catalyst used is zinc acetate and antimony trioxide.
Complete step by step answer:
Terylene is a condensation polymer of ethylene glycol and Terephthalic acid
Terylene is the product of condensation polymerisation, In this polymerisation repetitive condensation takes place between two bi-functional monomers known as ethylene glycol and Terephthalic acid and produces high molecular mass condensation polymers. So it is prepared by the condensation polymerization of ethylene glycol and Terephthalic acid with removal of water. This reaction is performed at temperature 420-460 K in the presence of a catalyst such as zinc acetate and antimony trioxide.
Terylene is also called Dacron,Terylene is also called polyester.
Polymerisation reaction for the preparation of Terylene:
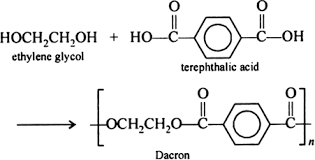
In the given 4 options, the only choice is Terephthalic acid it is because
Purified terephthalic acid available at lower cost and very easy and within less time direct esterification process can be done.
Properties of Terylene
Terylene is strong fibre and but this fibre loss its strength when it dipped in water
It is elastic in nature
Terylene can wash very easily and dries quickly.
It does not get damaged by acidic substances
Bleaches or dry cleaning agents cannot damage the terylene.
Use of Terylene-
Terylene used to prepare clothes like sarees, tapestry and dress material.
Note:
The Terylene is also called as Dacron and polyester and alcohol group in ethylene glycol and carboxylic acid group changes into ester group.
Complete step by step answer:
Terylene is a condensation polymer of ethylene glycol and Terephthalic acid
Terylene is the product of condensation polymerisation, In this polymerisation repetitive condensation takes place between two bi-functional monomers known as ethylene glycol and Terephthalic acid and produces high molecular mass condensation polymers. So it is prepared by the condensation polymerization of ethylene glycol and Terephthalic acid with removal of water. This reaction is performed at temperature 420-460 K in the presence of a catalyst such as zinc acetate and antimony trioxide.
Terylene is also called Dacron,Terylene is also called polyester.
Polymerisation reaction for the preparation of Terylene:

In the given 4 options, the only choice is Terephthalic acid it is because
Purified terephthalic acid available at lower cost and very easy and within less time direct esterification process can be done.
Properties of Terylene
Terylene is strong fibre and but this fibre loss its strength when it dipped in water
It is elastic in nature
Terylene can wash very easily and dries quickly.
It does not get damaged by acidic substances
Bleaches or dry cleaning agents cannot damage the terylene.
Use of Terylene-
Terylene used to prepare clothes like sarees, tapestry and dress material.
Note:
The Terylene is also called as Dacron and polyester and alcohol group in ethylene glycol and carboxylic acid group changes into ester group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




