
Why is tartaric acid added to baking soda to get baking powder?
Answer
528.3k+ views
Hint: We have to remember that the tartaric acid is usually present in fruits such as banana, citrus and many more. It is an organic acid since it contains carbon atoms in it. It is called acid as it has a carboxyl group present in it. This acid is found to be non-toxic. Baking soda has a chemical name which is sodium bicarbonate.
Complete answer:
Now we can draw the structure of tartaric acid as,
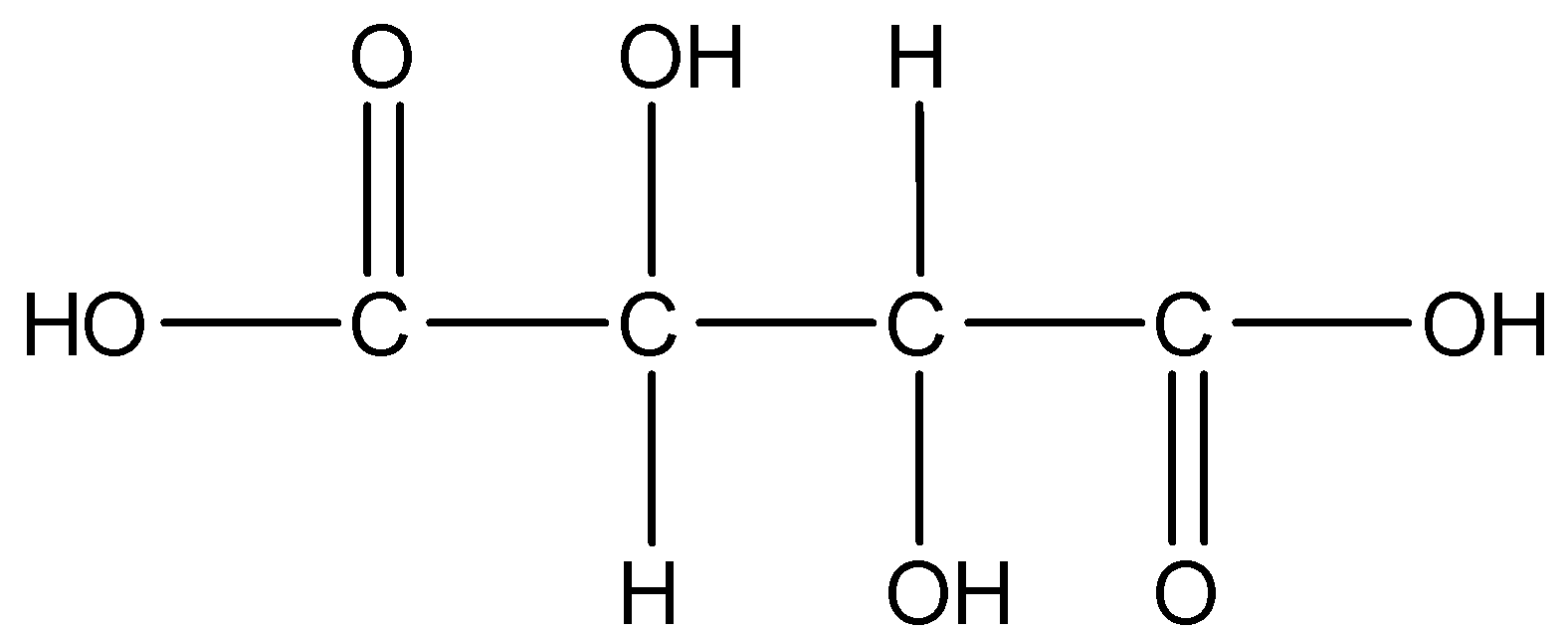
Since baking soda is basic in nature as it contains bicarbonate ions in it with sodium salt. So to neutralize the excess alkalinity in baking soda tartaric acid is added so as to neutralize the product i.e. baking powder. Also there is one more reason for adding tartaric acid into baking soda to get baking powder is that the when sodium bicarbonate decomposes itself into carbonate salt heat is released and to neutralize that excess heat an acid which is tartaric acid is added to it in order to make the system neutral and to get baking powder.
\[NaHC{O_3} \to N{a_2}C{O_3} + C{O_2} + {H_2}O\]
\[N{a_2}C{O_3} + Tartaricacid \to \] Neutralize the bitterness which is produced by \[N{a_2}C{O_3}\]sodium bicarbonate.
Note:
We have to remember that the baking powder is used in making cakes and other bakery products. When a base and an acid react together they form a neutral salt and water. Also, alkaline substances have bitter taste in order to overcome that taste an acid is added. We need to know that tartaric acid is used as it is mild edible acid which is present in citrus fruits. Also this acid is here working in converting baking soda into baking powder.
Complete answer:
Now we can draw the structure of tartaric acid as,

Since baking soda is basic in nature as it contains bicarbonate ions in it with sodium salt. So to neutralize the excess alkalinity in baking soda tartaric acid is added so as to neutralize the product i.e. baking powder. Also there is one more reason for adding tartaric acid into baking soda to get baking powder is that the when sodium bicarbonate decomposes itself into carbonate salt heat is released and to neutralize that excess heat an acid which is tartaric acid is added to it in order to make the system neutral and to get baking powder.
\[NaHC{O_3} \to N{a_2}C{O_3} + C{O_2} + {H_2}O\]
\[N{a_2}C{O_3} + Tartaricacid \to \] Neutralize the bitterness which is produced by \[N{a_2}C{O_3}\]sodium bicarbonate.
Note:
We have to remember that the baking powder is used in making cakes and other bakery products. When a base and an acid react together they form a neutral salt and water. Also, alkaline substances have bitter taste in order to overcome that taste an acid is added. We need to know that tartaric acid is used as it is mild edible acid which is present in citrus fruits. Also this acid is here working in converting baking soda into baking powder.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




