
Suppose following reaction:
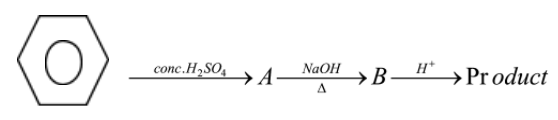
The structure of main product will be:
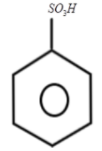
1.
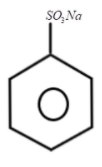
2.
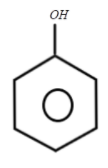
3.




Answer
573.3k+ views
Hint:As we all know that organic chemistry is full of dozens of chemical reactions, so to tackle these reactions, remember the working and functioning of reagents and the reactants to solve for the product. Reagents can be broken into ions to simplify and check whether reduction or oxidation is happening.
Complete answer:
The secret to solve such a type of question is simple, first look at the pattern like which functional group is involved in the reaction and which reagents do you have to proceed for the further solution. Whether an oxidizing agent is present or any reducing agent is present. So let us look at the first reaction, we know that fuming concentrated sulphuric acid when reacts with benzene it results in the formation of benzene sulphonic acid, sulphur will act as an electrophile and thus benzene attacks the sulphur and produces benzene sulphonic acid which we can represent as:
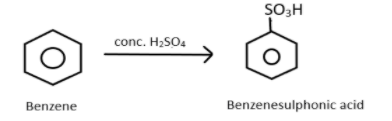
Now, this benzenesulfonic acid will react with the next reagent which is NaOH and when heated it will give sodium phenoxide ion which can represented as:
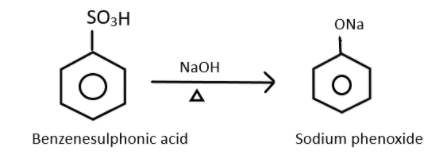
Finally after the formation of sodium phenoxide, the product will now react with sulphuric acid which will decompose it into phenol. This can be represented as:
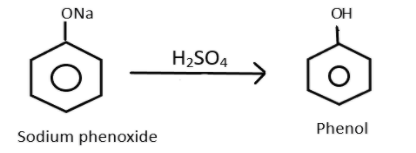
Therefore, we get to know that the last product which is formed is Phenol and overall reaction can be represented as:
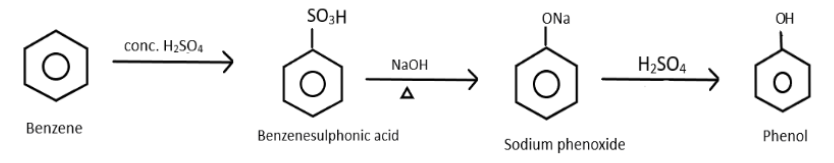
Hence, the correct answer is (3).
Note:
Always remember that oxidation of alcohols gives aldehydes and oxidation of aldehydes results in carboxylic acids and reverse is the case for reduction and benzene sulfonation and nitration will take place in the presence of concentrated sulphuric acid and nitric acid respectively.
Complete answer:
The secret to solve such a type of question is simple, first look at the pattern like which functional group is involved in the reaction and which reagents do you have to proceed for the further solution. Whether an oxidizing agent is present or any reducing agent is present. So let us look at the first reaction, we know that fuming concentrated sulphuric acid when reacts with benzene it results in the formation of benzene sulphonic acid, sulphur will act as an electrophile and thus benzene attacks the sulphur and produces benzene sulphonic acid which we can represent as:

Now, this benzenesulfonic acid will react with the next reagent which is NaOH and when heated it will give sodium phenoxide ion which can represented as:

Finally after the formation of sodium phenoxide, the product will now react with sulphuric acid which will decompose it into phenol. This can be represented as:

Therefore, we get to know that the last product which is formed is Phenol and overall reaction can be represented as:

Hence, the correct answer is (3).
Note:
Always remember that oxidation of alcohols gives aldehydes and oxidation of aldehydes results in carboxylic acids and reverse is the case for reduction and benzene sulfonation and nitration will take place in the presence of concentrated sulphuric acid and nitric acid respectively.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




