
Sucrose on hydrolysis gives?
(A) Glucose + Glucose
(B) Glucose + Galactose
(C) Glucose + Fructose
(D) Glucose + Lactose
Answer
590.1k+ views
Hint: Sucrose is a disaccharide made by the reaction between two aldohexose. Draw the expanded structure of sucrose. Now try to split the complex structure into two monosaccharides such that both compounds have 6 carbon atoms. Now identify the compounds obtained and then write the IUPAC name for the same.
Complete step by step answer:
Sucrose is considered as common sugar. Sucrose is produced naturally in plants from which the edible table sugar is refined. The molecular formula for sucrose is ${{C}_{12}}{{H}_{22}}{{O}_{11}}$. We will now draw the expanded structure of a sucrose molecule.
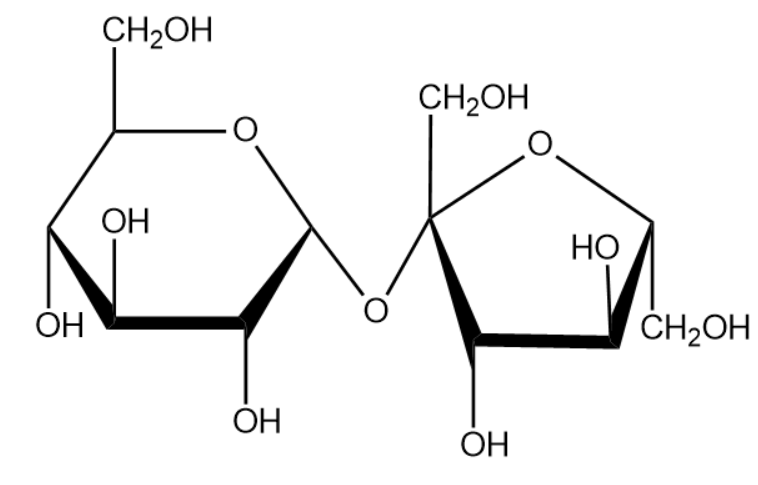
From the above structure we can identify the monosaccharide that help to from the compound sucrose. The structure compounds are given below:
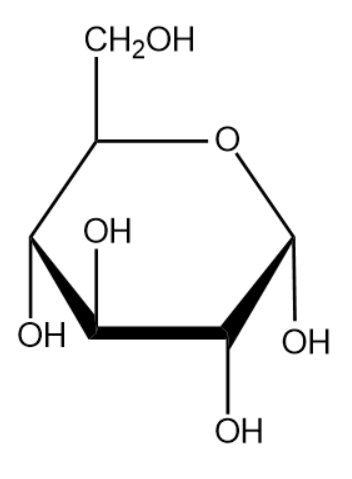
The name of the above monosaccharide is glucose.
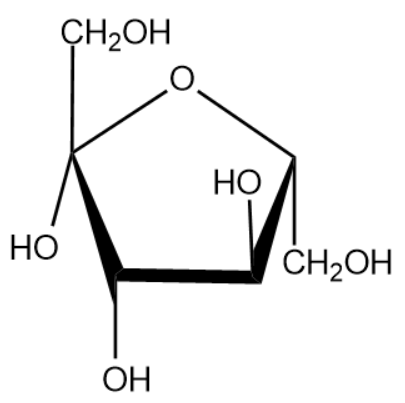
The name of the above monosaccharide is fructose.
From the above statements we can conclude that sucrose on hydrolysis will give the respective monosaccharides, glucose and fructose. So, the correct answer is “Option C”.
Note: It is important to know that sucrose is dextrorotatory in nature. However, upon hydrolysis the products obtained are dextrorotatory glucose and laevorotatory fructose. Since the rotation of fructose is much more than that to glucose, the mixture as a whole is considered laevorotatory. Since, the hydrolysis of sucrose brings a significant change in the type of rotation to the mixture, i.e. from dextrorotatory to laevorotatory, it is also called as invert sugar.
Complete step by step answer:
Sucrose is considered as common sugar. Sucrose is produced naturally in plants from which the edible table sugar is refined. The molecular formula for sucrose is ${{C}_{12}}{{H}_{22}}{{O}_{11}}$. We will now draw the expanded structure of a sucrose molecule.

From the above structure we can identify the monosaccharide that help to from the compound sucrose. The structure compounds are given below:

The name of the above monosaccharide is glucose.

The name of the above monosaccharide is fructose.
From the above statements we can conclude that sucrose on hydrolysis will give the respective monosaccharides, glucose and fructose. So, the correct answer is “Option C”.
Note: It is important to know that sucrose is dextrorotatory in nature. However, upon hydrolysis the products obtained are dextrorotatory glucose and laevorotatory fructose. Since the rotation of fructose is much more than that to glucose, the mixture as a whole is considered laevorotatory. Since, the hydrolysis of sucrose brings a significant change in the type of rotation to the mixture, i.e. from dextrorotatory to laevorotatory, it is also called as invert sugar.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




