
How many structure isomers are possible in diphenylmethane (\[{{\text{C}}_{{\text{13}}}}{{\text{H}}_{{\text{12}}}}\]) when one of the hydrogen is replaced by a chlorine atom?
A) 4
B) 6
C) 7
D) 8
Answer
580.2k+ views
Hint: Structural isomers are compounds having the same number of atoms of each element, but have a different arrangement of the atoms. Many numbers of structural isomers are possible when all the atoms are the same in the molecule.
Complete step by step solution:
Diphenylmethane has the molecular formula \[{{\text{C}}_{{\text{13}}}}{{\text{H}}_{{\text{12}}}}\]. It is a simple alkane, methane, where the two hydrogen atoms are substituted by phenyl rings and its structure will be as follows;
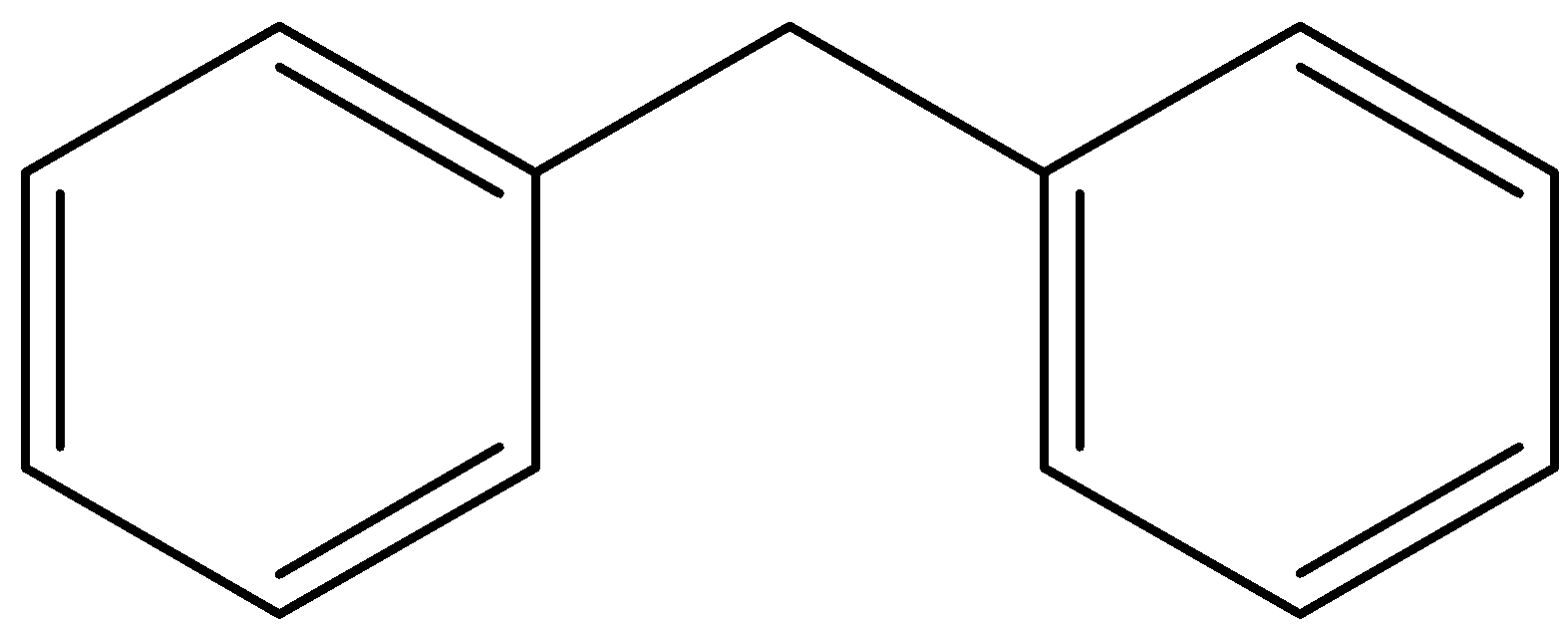
Here we have to substitute one hydrogen atom with a chlorine atom and then the structure will be as follows;

Here we have the same number of atoms in the structural isomers but only the position of the atoms does change. Here the substituted chlorine atom can have four positions and they are the ortho, meta and para positions of the substituted benzene ring and the final one is by substituting one hydrogen in the central \[{\text{ - C}}{{\text{H}}_{\text{2}}}\] bond. The four structures can be depicted as follows;
1)
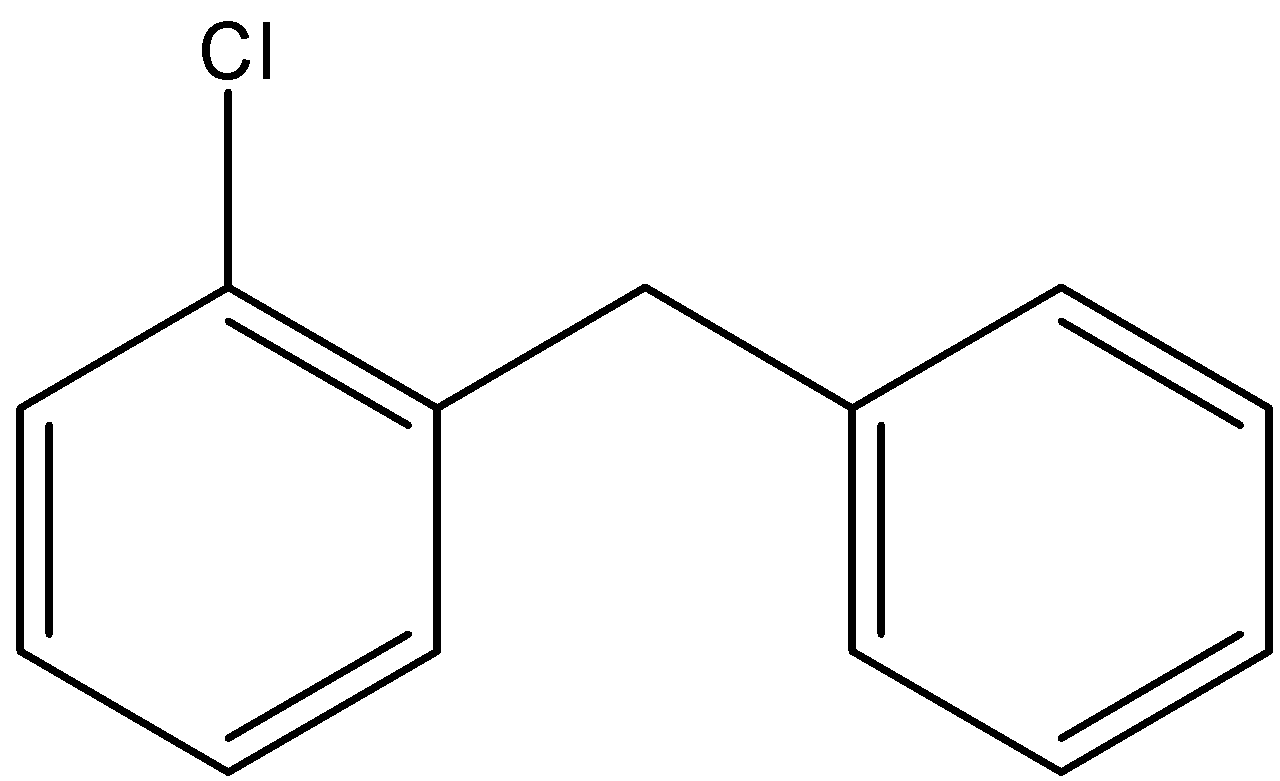
2)

3)
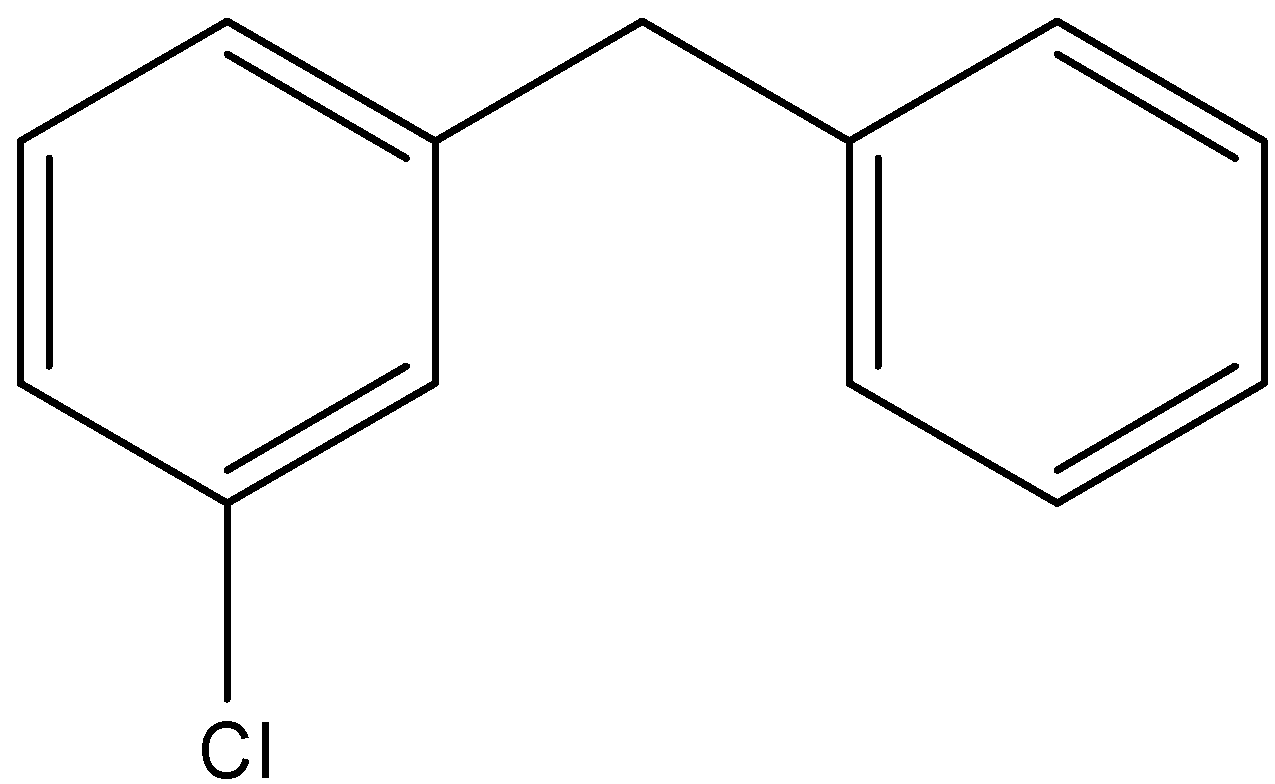
4)
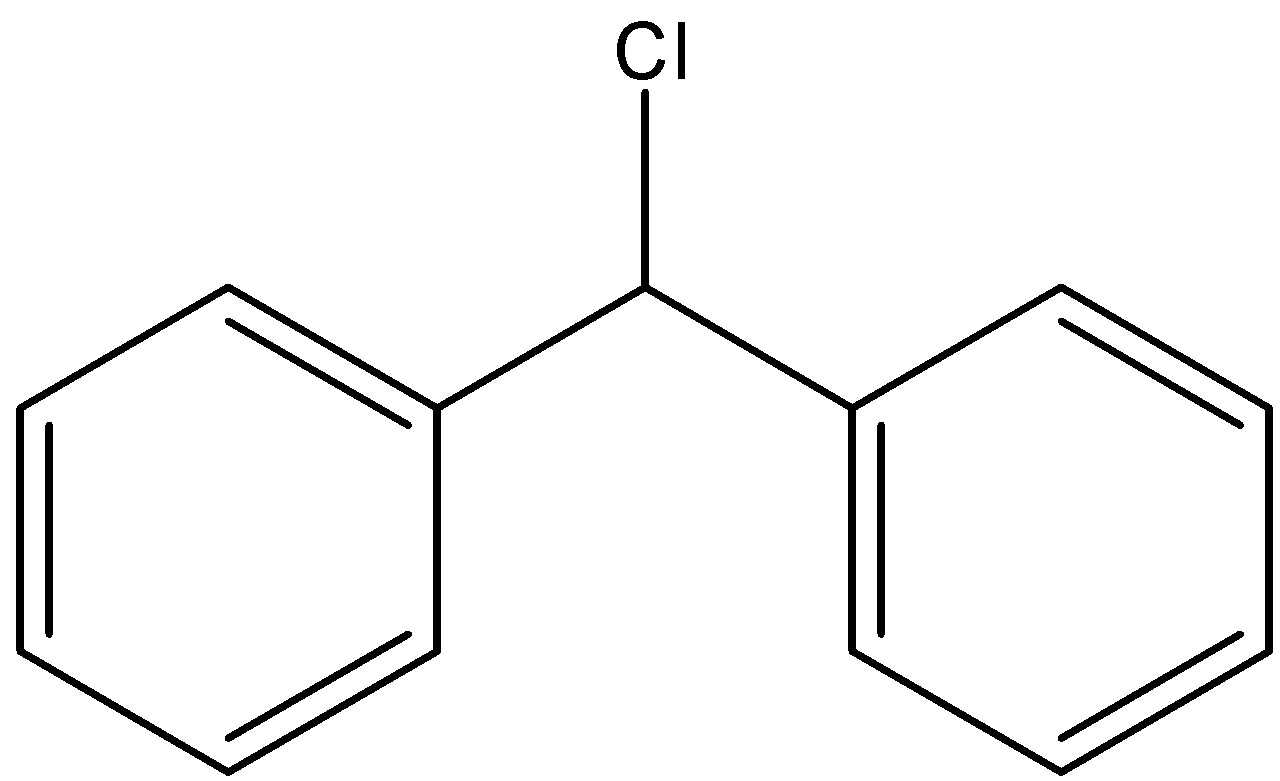
Therefore, we see that four structural isomers are possible for diphenylmethane whose one hydrogen atom is substituted by a chlorine atom.
So, the correct answer is Option A.
Additional Information:
Diphenylmethane is a white solid. It is prepared by the Friedel-Crafts alkylation of benzyl chloride with benzene in the presence of Lewis Acid Catalyst like Aluminium Chloride.
Note: Isomers are compounds having the same molecular formula but different structures. They can be structural isomers, position isomers etc. The isomers are created by the different orientations of the constituent atoms.
Complete step by step solution:
Diphenylmethane has the molecular formula \[{{\text{C}}_{{\text{13}}}}{{\text{H}}_{{\text{12}}}}\]. It is a simple alkane, methane, where the two hydrogen atoms are substituted by phenyl rings and its structure will be as follows;

Here we have to substitute one hydrogen atom with a chlorine atom and then the structure will be as follows;

Here we have the same number of atoms in the structural isomers but only the position of the atoms does change. Here the substituted chlorine atom can have four positions and they are the ortho, meta and para positions of the substituted benzene ring and the final one is by substituting one hydrogen in the central \[{\text{ - C}}{{\text{H}}_{\text{2}}}\] bond. The four structures can be depicted as follows;
1)

2)

3)

4)

Therefore, we see that four structural isomers are possible for diphenylmethane whose one hydrogen atom is substituted by a chlorine atom.
So, the correct answer is Option A.
Additional Information:
Diphenylmethane is a white solid. It is prepared by the Friedel-Crafts alkylation of benzyl chloride with benzene in the presence of Lewis Acid Catalyst like Aluminium Chloride.
Note: Isomers are compounds having the same molecular formula but different structures. They can be structural isomers, position isomers etc. The isomers are created by the different orientations of the constituent atoms.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




