
What is the structural formula of fumaric acid?
A.
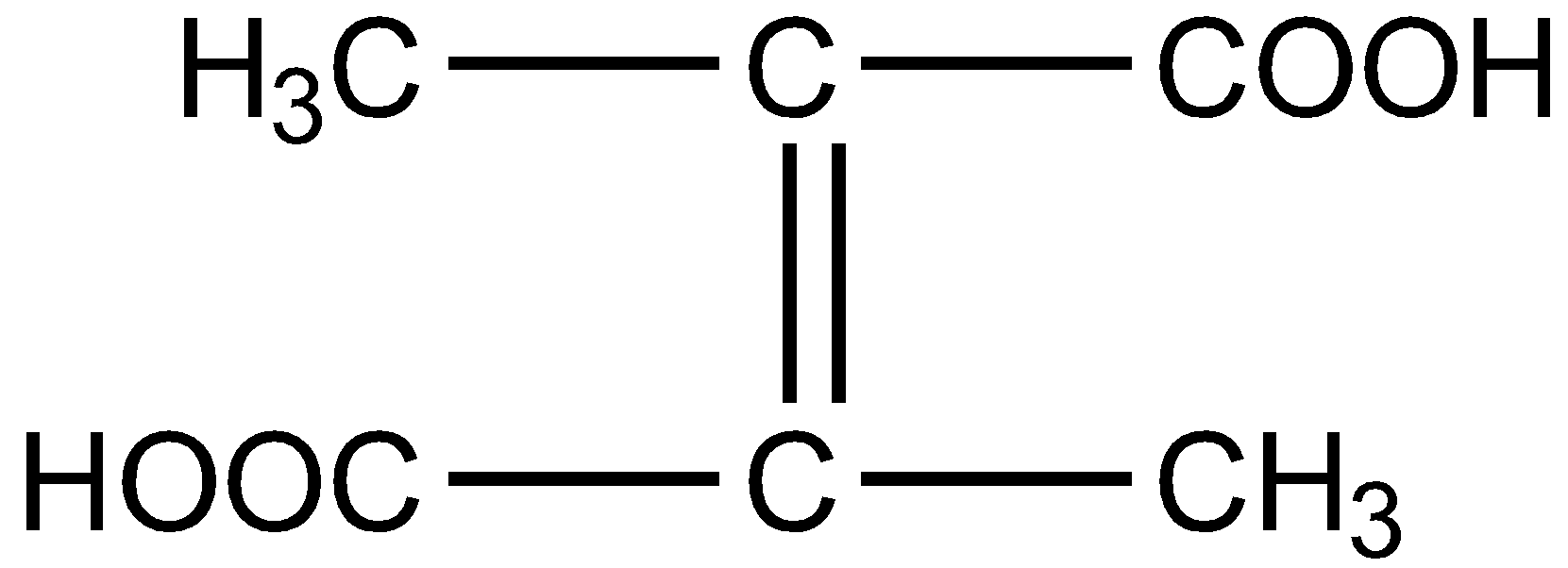
B.
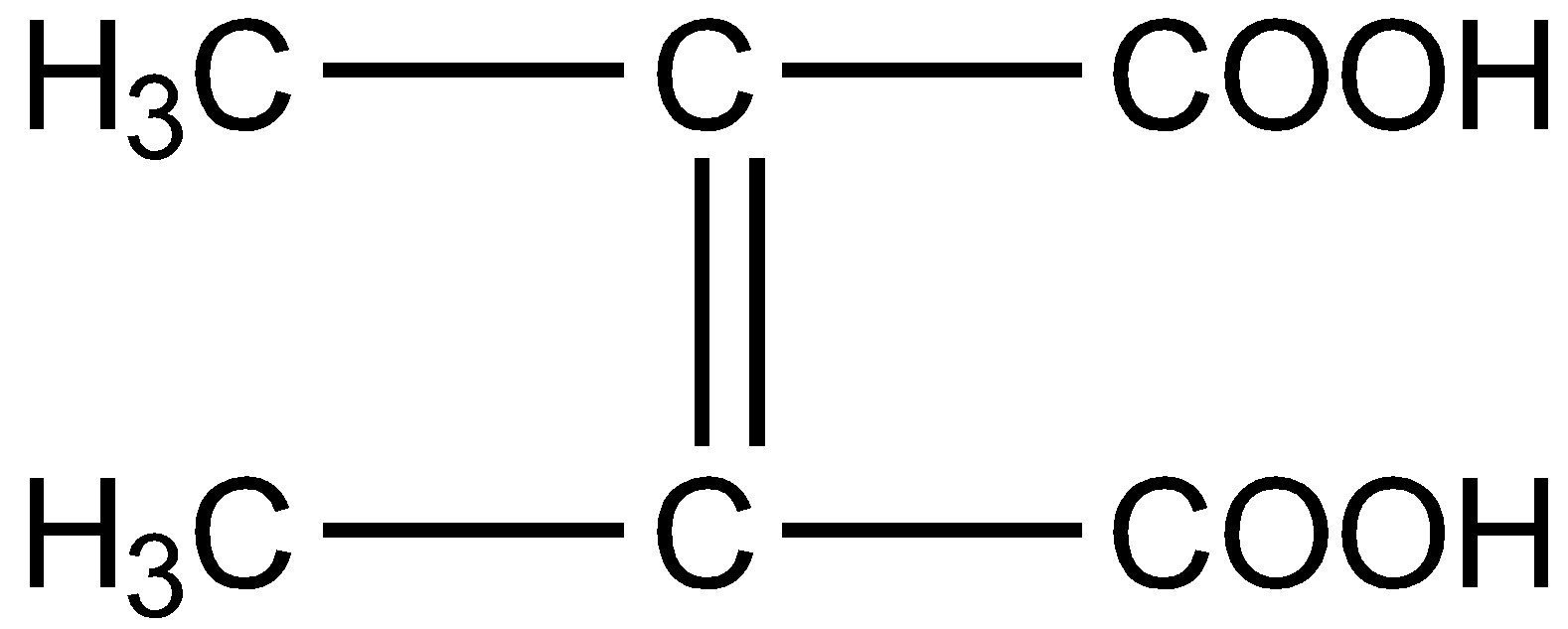
C.
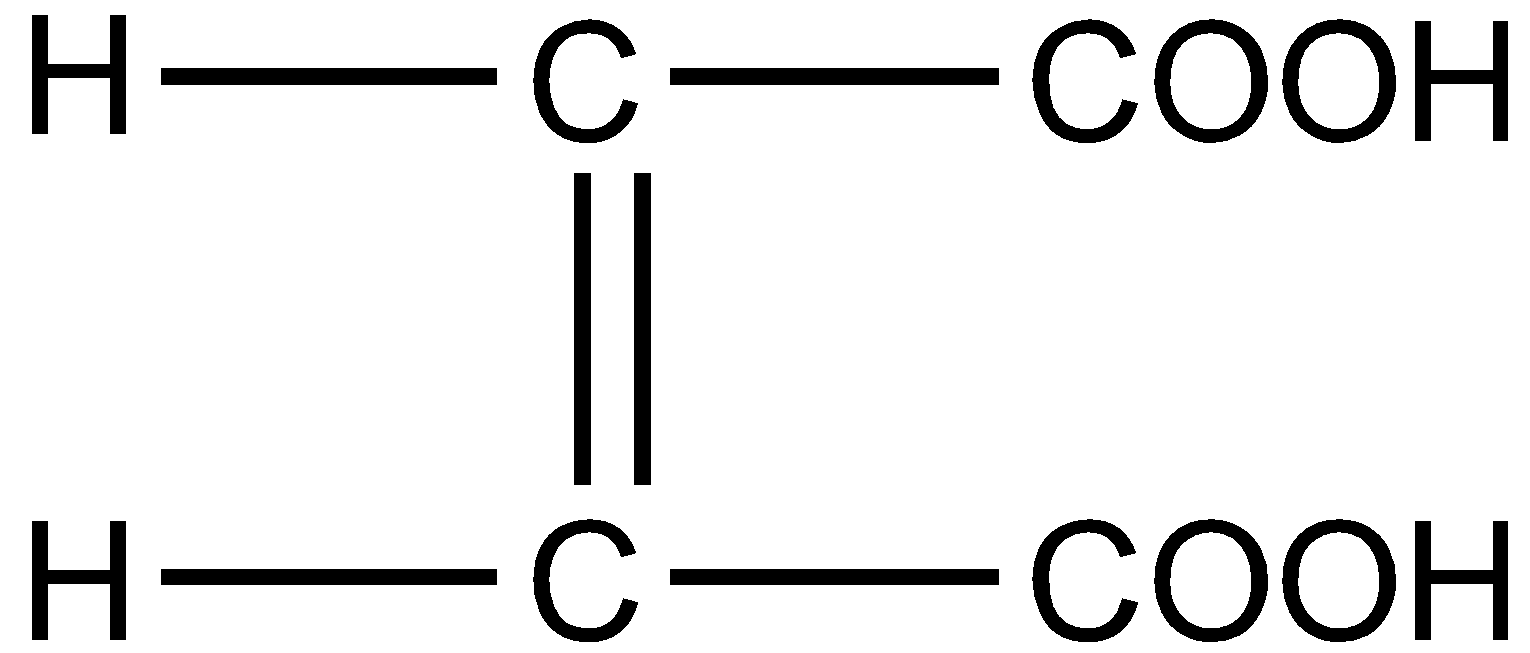
D.
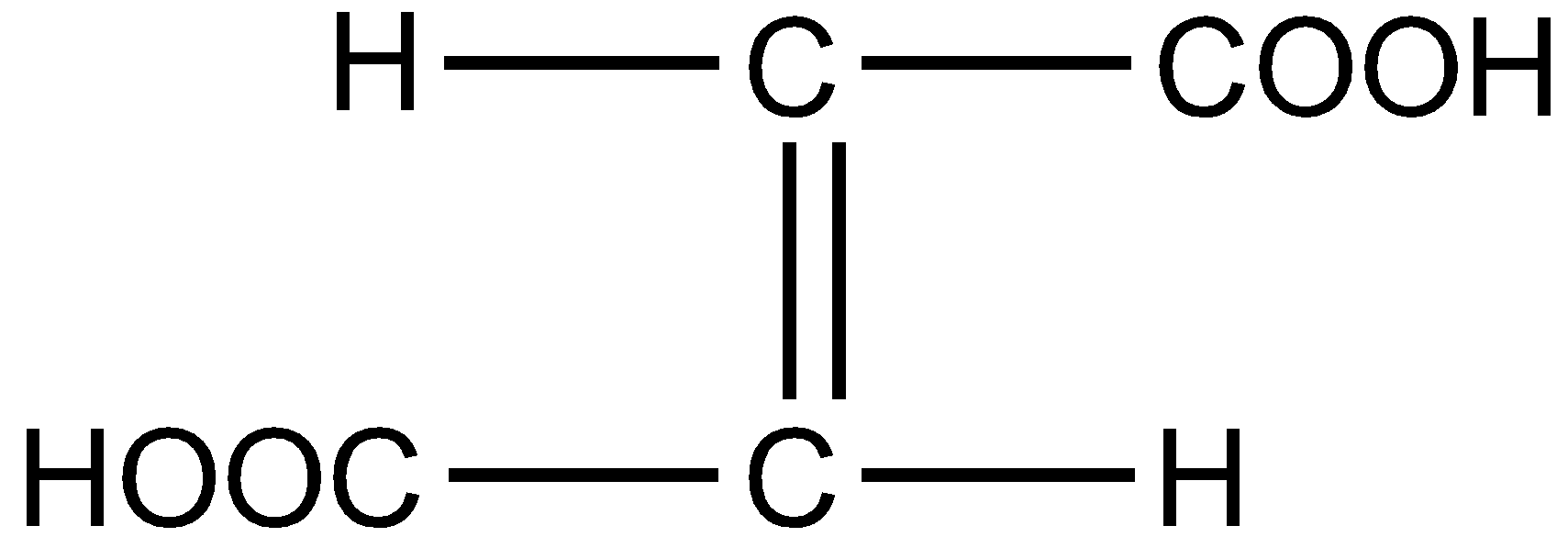




Answer
566.7k+ views
Hint: Fumaric acid is also known as E-Butenedioic acid as per IUPAC nomenclature and it is used as a food additive. It is widely available in nature and the salts and esters of fumaric acid are known as fumarates.
Complete step by step answer:
Fumaric acid also known as 2-Butenedioic acid or trans-1,2-Ethylene-dicarboxylic acid is a trans-isomer of symmetric, unsaturated dicarboxylic acid. The chemical formula is ${C_4}{H_4}{O_4}$. The structural formula for fumaric acid is written as :
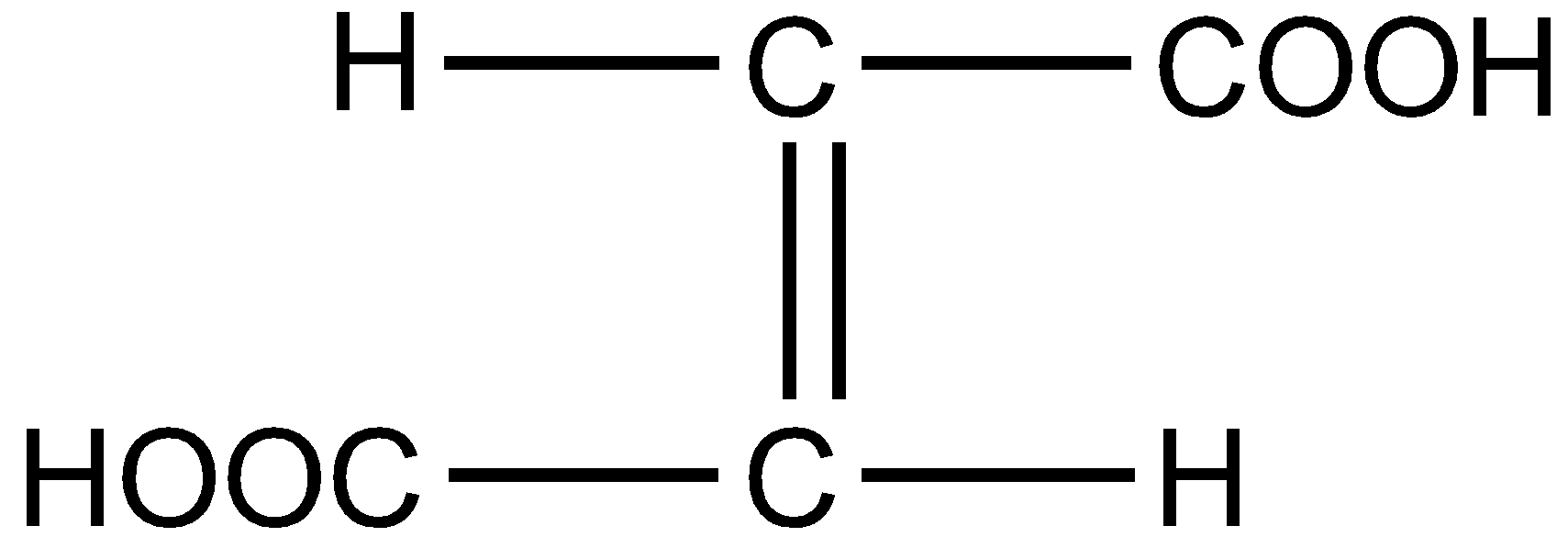
Hence the correct answer is option D.
At present, Fumaric acid is produced through the isomerization of maleic acid.
Additional information:
Fumaric acid is one of the most economical solid food acids as it is required in less quantity and also it has less cost. It is also used to increase the gel strength for gelatins and it does not give a strong acid-like taste instead provides the taste of grape. The esters of fumaric acids have medicinal use and they are used in the treatment of various skin disorders and psoriasis. Fumaric acid is less soluble in water at different temperatures and its usage in soft drinks is not allowed in the UK.
Note:
Fumaric acid is more acidic than citric acid and is mostly produced by petroleum-based chemical synthesis. It is a colorless, crystalline powder with a fruit-like taste. Its cis isomer is known as maleic acid. It is used to extend the shelf life for food items as they inactivate foodborne pathogens. Also work is in progress for the development of bio-based fumaric acid from renewable resources.
Complete step by step answer:
Fumaric acid also known as 2-Butenedioic acid or trans-1,2-Ethylene-dicarboxylic acid is a trans-isomer of symmetric, unsaturated dicarboxylic acid. The chemical formula is ${C_4}{H_4}{O_4}$. The structural formula for fumaric acid is written as :

Hence the correct answer is option D.
At present, Fumaric acid is produced through the isomerization of maleic acid.
Additional information:
Fumaric acid is one of the most economical solid food acids as it is required in less quantity and also it has less cost. It is also used to increase the gel strength for gelatins and it does not give a strong acid-like taste instead provides the taste of grape. The esters of fumaric acids have medicinal use and they are used in the treatment of various skin disorders and psoriasis. Fumaric acid is less soluble in water at different temperatures and its usage in soft drinks is not allowed in the UK.
Note:
Fumaric acid is more acidic than citric acid and is mostly produced by petroleum-based chemical synthesis. It is a colorless, crystalline powder with a fruit-like taste. Its cis isomer is known as maleic acid. It is used to extend the shelf life for food items as they inactivate foodborne pathogens. Also work is in progress for the development of bio-based fumaric acid from renewable resources.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




