
What is the structural formula for Phosphinic acid?
Answer
525.3k+ views
Hint: Structural formula of any compound is the graphical representation of the compound’s molecular structure. It shows how atoms are possibly arranged in three-dimensional space. It also shows the chemical bonding within the molecule.
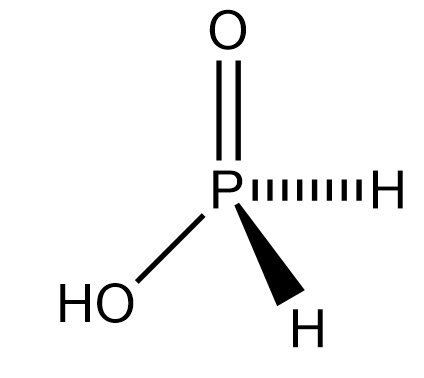
Complete answer: Phosphinic acid also called Hypophosphorous acid contains a phosphorus atom covalently bound to two hydrogen atoms, a hydroxyl group and one oxygen atom. It is also called phosphorous oxoacid.
The molecular formula of phosphinic acid is \[{H_3}P{O_2}\]. To get the structural formula we will simply create a graphical representation of the molecular formula. Hence the structural formula of phosphinic acid is:
Additional Information:
Phosphinic acid acts as a powerful reducing agent. It is a colourless compound and has a low melting point. It is soluble in water, dioxane and alcohols. The molecular formula of phosphinic acid can also be written in a more descriptive way as \[HOP(O){H_2}\]. Phosphinic acid is a conjugate acid of phosphinate. Salts obtained from phosphinic acid are called hypophosphites. It has a primary use in the electroless nickel plating.
Note:
When writing the structural formula, first try to write its molecular formula in a descriptive way as it will tell you how elements are linked with each other. This descriptive formula is also called condensed structural formula.
In the structural formula, the number of short lines indicate whether the bond is a single, double, or triple covalent bond. One small line indicates a single bond, two small lines indicate double bonds and three small lines indicate triple bonds.
Complete answer: Phosphinic acid also called Hypophosphorous acid contains a phosphorus atom covalently bound to two hydrogen atoms, a hydroxyl group and one oxygen atom. It is also called phosphorous oxoacid.
The molecular formula of phosphinic acid is \[{H_3}P{O_2}\]. To get the structural formula we will simply create a graphical representation of the molecular formula. Hence the structural formula of phosphinic acid is:

Additional Information:
Phosphinic acid acts as a powerful reducing agent. It is a colourless compound and has a low melting point. It is soluble in water, dioxane and alcohols. The molecular formula of phosphinic acid can also be written in a more descriptive way as \[HOP(O){H_2}\]. Phosphinic acid is a conjugate acid of phosphinate. Salts obtained from phosphinic acid are called hypophosphites. It has a primary use in the electroless nickel plating.
Note:
When writing the structural formula, first try to write its molecular formula in a descriptive way as it will tell you how elements are linked with each other. This descriptive formula is also called condensed structural formula.
In the structural formula, the number of short lines indicate whether the bond is a single, double, or triple covalent bond. One small line indicates a single bond, two small lines indicate double bonds and three small lines indicate triple bonds.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




