
State Gay Lusaacc's law of gaseous volume
Answer
587.1k+ views
Hint: The Gay Lusaacc’s law of gaseous volume establishes a relationship between pressure and temperature of a fixed mass of gas kept at a constant volume. Knowing the basic aim of the Gay Lusaacc’s law helps in stating the law.
Complete step by step answer:
The Gay Lusaacc’s law of gaseous volume states that the pressure of a given mass of gas varies directly with the absolute temperature, when the volume is kept constant.
In simple terms we can explain that when gases react together they do so in volume which bears a simple whole number ratio provided that the temperature and the pressure of the reacting gases and their products remain constant.
Mathematically it can be written as,
P/T = k
Where, P is the pressure is the exerted by the gas
T is the absolute temperature of the gas
k is the constant
The relationship between the pressure and absolute temperature of a given mass of gas at constant volume can be illustrated graphically as follows:
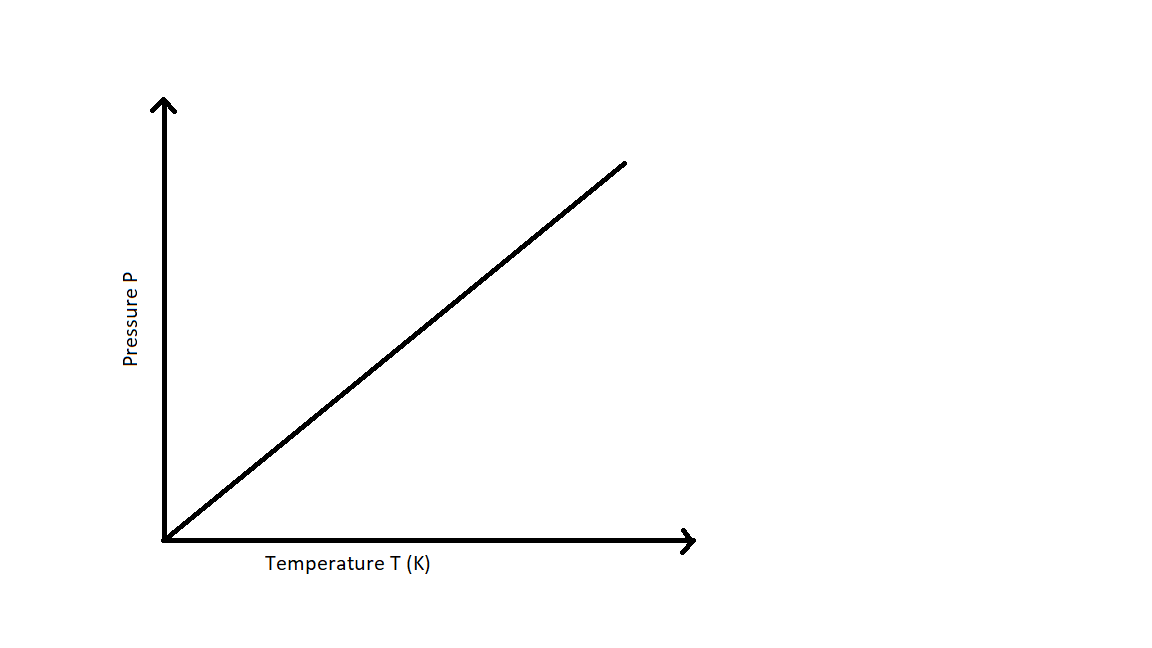
From the graph it can be understood that the pressure of the gas kept at constant volume reduces constantly as it is cooled until the gas eventually undergoes condensation and becomes a liquid.
Note: The Gay Lusaacc’s law can be explained with the help of an example such as in the chemical reaction of formation of hydrogen chloride gas, one volume of hydrogen is mixed with two volumes of chlorine. This results in the formation of two molecules of hydrogen chloride at constant temperature and pressure.
Complete step by step answer:
The Gay Lusaacc’s law of gaseous volume states that the pressure of a given mass of gas varies directly with the absolute temperature, when the volume is kept constant.
In simple terms we can explain that when gases react together they do so in volume which bears a simple whole number ratio provided that the temperature and the pressure of the reacting gases and their products remain constant.
Mathematically it can be written as,
P/T = k
Where, P is the pressure is the exerted by the gas
T is the absolute temperature of the gas
k is the constant
The relationship between the pressure and absolute temperature of a given mass of gas at constant volume can be illustrated graphically as follows:

From the graph it can be understood that the pressure of the gas kept at constant volume reduces constantly as it is cooled until the gas eventually undergoes condensation and becomes a liquid.
Note: The Gay Lusaacc’s law can be explained with the help of an example such as in the chemical reaction of formation of hydrogen chloride gas, one volume of hydrogen is mixed with two volumes of chlorine. This results in the formation of two molecules of hydrogen chloride at constant temperature and pressure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




