
Specify the coordination geometry and hybridization of $N$ and $B$ atoms in $1:1$ complex of $B{F_3}$ and $N{H_3}$ .
A.$N:tetrahedral,s{p^3};B:planar,s{p^2}$
B.$N:pyramidal,s{p^3};B:pyramidal,s{p^3}$
C.$N:pyramidal,s{p^3};B:planar,s{p^3}$
D.$N:tetrahedral,s{p^3};B:tetrahedral,s{p^3}$
Answer
517.2k+ views
Hint: We have to know that, the metal-ligand bonds are viewed as facilitating covalent bonds in which electron sets from the given ligand enter empty electron orbitals on the focal iota. Hybridization is summoned since they facilitate bonds that are framed and are unclear from one another.
Complete answer:
We have to know that, the Coordination number of a particle in a given particle or a precious stone alludes to the complete number of iotas, particles, or atoms clung to the molecule being referred to ligancy is another term used to allude to the coordination number of a molecule. The atoms, particles, or atoms that are clung to the focal particle are called ligands. The ligancy of particles is determined diversely when contrasted with computing the coordination number of a focal molecule in a precious stone.
We have to know that hybridization is characterized as the idea of blending two nuclear orbitals with similar energy levels to give a deteriorated new kind of orbitals. This intermixing depends on quantum mechanics. The nuclear orbitals of a similar energy level can just participate in hybridization and both full-filled and half-filled orbitals can likewise partake in this interaction, if they have equivalent energy.
During the interaction of hybridization, the nuclear orbitals of comparative energy are combined as one like the blending of two $s$ orbitals or two $p$ orbitals or blending of an $s$ orbital with $p$ orbital or $s$ orbital with $d$ orbital.
Therefore, the structure of the given compounds are given,
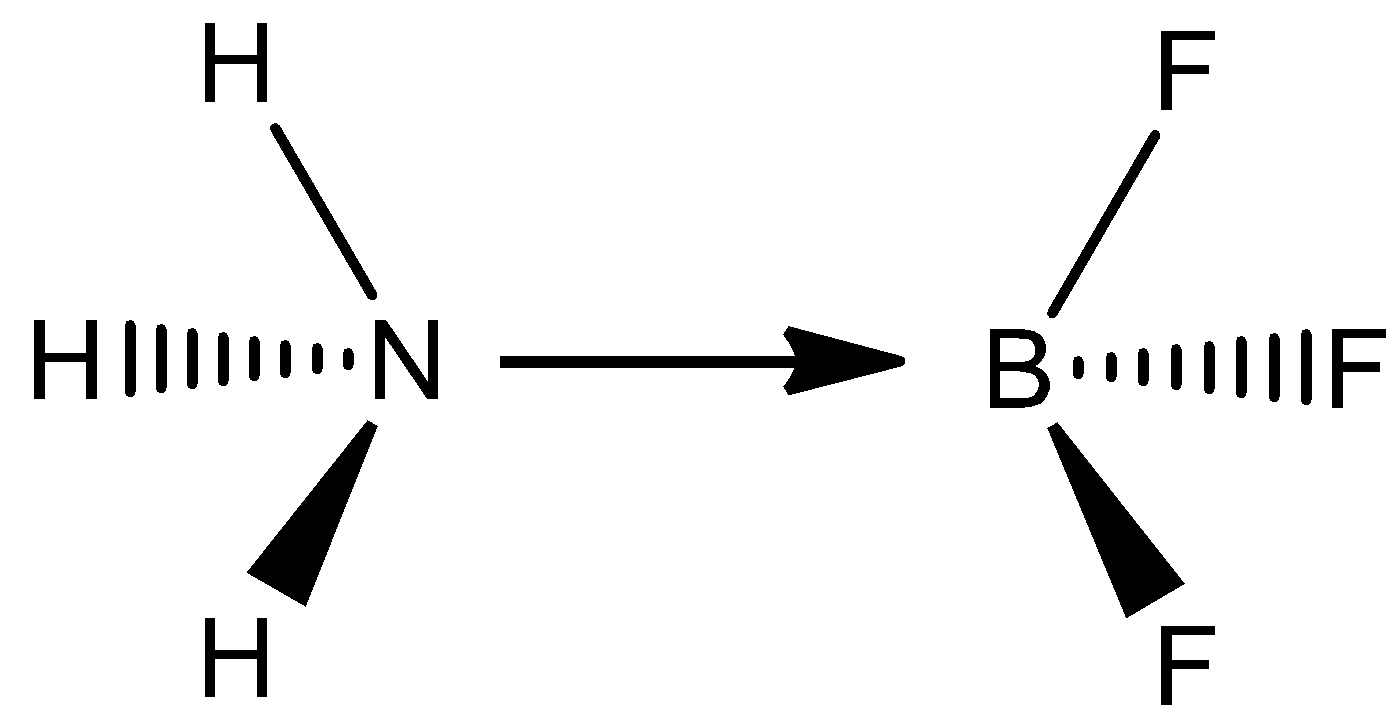
In a $1:1$ complex of $B{F_3}$ and $N{H_3}$ , the $N$ molecule has tetrahedral calculation with $s{p^3}$ hybridization and the $B$ atom has tetrahedral math with $s{p^3}$ hybridization.
Hence, the correct option is (D) $N:tetrahedral,s{p^3};B:tetrahedral,s{p^3}$ .
Note:
We have to know that, the communications between the nuclear orbitals of two unique particles result in sub-atomic orbitals, though when the nuclear orbitals of a similar molecule connect they structure crossover orbitals.
Complete answer:
We have to know that, the Coordination number of a particle in a given particle or a precious stone alludes to the complete number of iotas, particles, or atoms clung to the molecule being referred to ligancy is another term used to allude to the coordination number of a molecule. The atoms, particles, or atoms that are clung to the focal particle are called ligands. The ligancy of particles is determined diversely when contrasted with computing the coordination number of a focal molecule in a precious stone.
We have to know that hybridization is characterized as the idea of blending two nuclear orbitals with similar energy levels to give a deteriorated new kind of orbitals. This intermixing depends on quantum mechanics. The nuclear orbitals of a similar energy level can just participate in hybridization and both full-filled and half-filled orbitals can likewise partake in this interaction, if they have equivalent energy.
During the interaction of hybridization, the nuclear orbitals of comparative energy are combined as one like the blending of two $s$ orbitals or two $p$ orbitals or blending of an $s$ orbital with $p$ orbital or $s$ orbital with $d$ orbital.
Therefore, the structure of the given compounds are given,

In a $1:1$ complex of $B{F_3}$ and $N{H_3}$ , the $N$ molecule has tetrahedral calculation with $s{p^3}$ hybridization and the $B$ atom has tetrahedral math with $s{p^3}$ hybridization.
Hence, the correct option is (D) $N:tetrahedral,s{p^3};B:tetrahedral,s{p^3}$ .
Note:
We have to know that, the communications between the nuclear orbitals of two unique particles result in sub-atomic orbitals, though when the nuclear orbitals of a similar molecule connect they structure crossover orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




