
How many sigma bonds are present in caffeine?
Answer
559.2k+ views
Hint The concept of organic chemistry gives the answer where writing down the structure of caffeine which has molecular formula ${{C}_{8}}{{H}_{10}}{{N}_{4}}{{O}_{2}}$ and then calculating the number of sigma bonds will give the required answer.
Complete step – by – step answer:
From the previous chapters of chemistry, we have studied about the concepts of bond formation between two or more atoms to form a compound and also some of the related properties of the bonds that are associated with bond energy, bond order and length etc.
Let us now calculate the number of sigma bonds present in the caffeine based on its molecular formula and the structure.
- Caffeine is the chemical which is the stimulant in the central nervous system and it occurs naturally in the plant species of which cocoa beans, kola nuts, tea leaves and coffee beans are well known.
- Caffeine has the molecular formula ${{C}_{8}}{{H}_{10}}{{N}_{4}}{{O}_{2}}$ where it has two cyclic structures attached to each other that consist of the hetero atoms. The structure has one six membered ring fused with the one five membered ring and totally has alternate carbon and nitrogen bonds wherein two oxygen atoms are present on the two carbon atoms of the six membered ring as a carbonyl group.
Therefore, based on these facts, we can write the structure of caffeine as shown below,
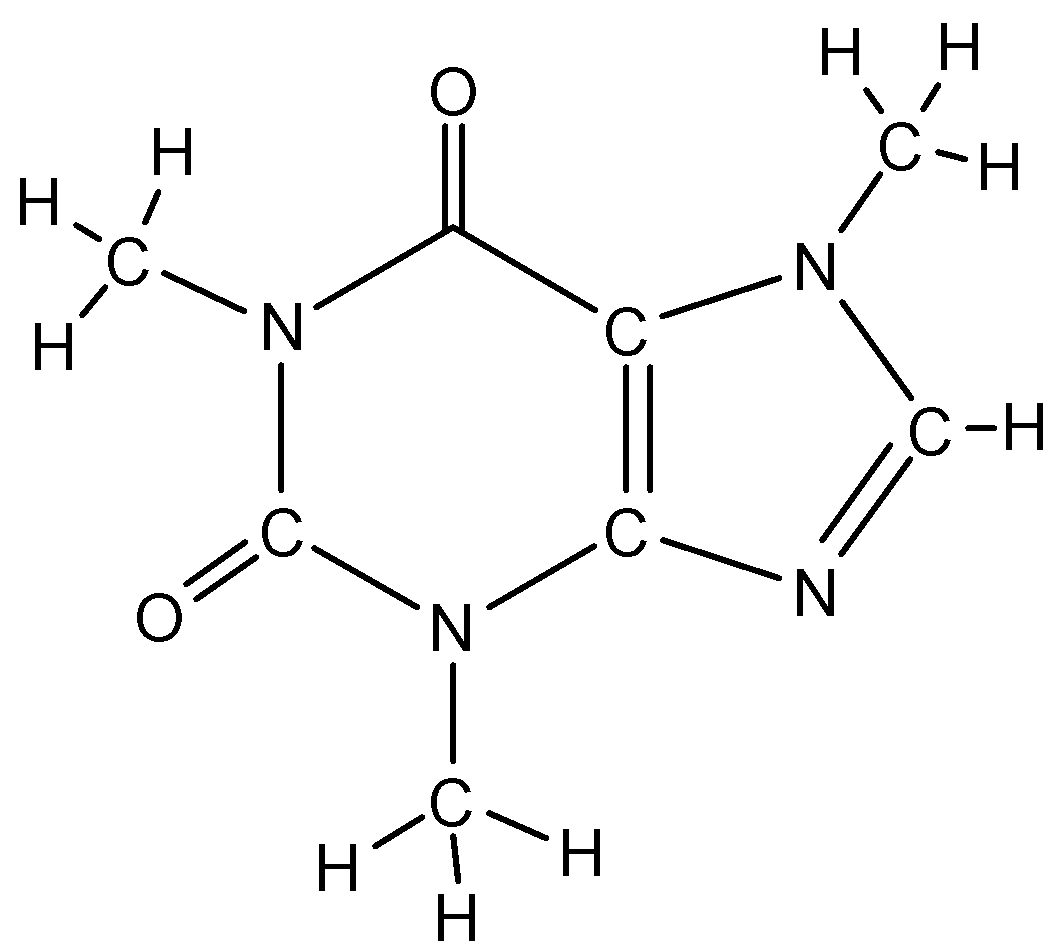
Therefore, by this structure, the total number of sigma bonds present is 25.
Note: You should remember the structures of basic chemicals and be thorough with the common names given for the chemical compounds along with their molecular formula and structures and this will help you to give the correct answer.
Complete step – by – step answer:
From the previous chapters of chemistry, we have studied about the concepts of bond formation between two or more atoms to form a compound and also some of the related properties of the bonds that are associated with bond energy, bond order and length etc.
Let us now calculate the number of sigma bonds present in the caffeine based on its molecular formula and the structure.
- Caffeine is the chemical which is the stimulant in the central nervous system and it occurs naturally in the plant species of which cocoa beans, kola nuts, tea leaves and coffee beans are well known.
- Caffeine has the molecular formula ${{C}_{8}}{{H}_{10}}{{N}_{4}}{{O}_{2}}$ where it has two cyclic structures attached to each other that consist of the hetero atoms. The structure has one six membered ring fused with the one five membered ring and totally has alternate carbon and nitrogen bonds wherein two oxygen atoms are present on the two carbon atoms of the six membered ring as a carbonyl group.
Therefore, based on these facts, we can write the structure of caffeine as shown below,

Therefore, by this structure, the total number of sigma bonds present is 25.
Note: You should remember the structures of basic chemicals and be thorough with the common names given for the chemical compounds along with their molecular formula and structures and this will help you to give the correct answer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




