
How many sigma and pi bonds are present in tetracyanoethylene?
A. Nine $$\sigma $$ and nine $$\Pi $$
B. Five $$\Pi $$ and nine $$\sigma $$
C. Nine $$\sigma $$and seven $$\Pi $$
D. Eight $$\sigma $$and eight $$\Pi $$
Answer
565.5k+ views
Hint: The pi bonds are defined as the bonds which are formed by the side wise overlapping of the atom orbital. The pi bonds are formed after the sigma bonding takes place. The sigma bond is formed by the axial overlapping of the atomic orbital.
Complete step by step answer:
The chemical; structure of the tetracyanoethylene is drawn below. The structure has 5 carbon atoms and four nitrogen atoms. There are three triple bonds, four single bonds and one double bond in the structure. As I told you earlier that pi bond is formed after sigma bond so the double bond will have one pi bond and triple bond will have two pi bonds.
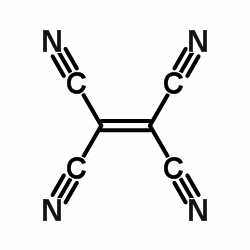
So after seeing the structure we get to know that the central carbon atoms attached to the side carbon are bonded by a single bond. So all single bonds are sigma bonds. So here singly bonded sigma bonds are 4. The double bonded sigma bond in this structure is 1 which is the central C-C bond. The triple bonded sigma bond in this structure is 4 which are carbon nitrogen bonds. So the total sigma bonds are 9 $$\sigma $$bonds. So now the pi bond in double bonded atoms is 1 that is the central C-C bond. The other 2 pi bonds in each triple bonded carbon nitrogen bonds are in total 8. So the total pi bonds are 9.
so the correct option for the question is option A.
Note: sigma bonds are more stronger than pi bonds because they hold the atoms rigidly in it. They also overlap the orbital directly. Due to which the electron density gets more concentrated between the two nuclei.
Complete step by step answer:
The chemical; structure of the tetracyanoethylene is drawn below. The structure has 5 carbon atoms and four nitrogen atoms. There are three triple bonds, four single bonds and one double bond in the structure. As I told you earlier that pi bond is formed after sigma bond so the double bond will have one pi bond and triple bond will have two pi bonds.

So after seeing the structure we get to know that the central carbon atoms attached to the side carbon are bonded by a single bond. So all single bonds are sigma bonds. So here singly bonded sigma bonds are 4. The double bonded sigma bond in this structure is 1 which is the central C-C bond. The triple bonded sigma bond in this structure is 4 which are carbon nitrogen bonds. So the total sigma bonds are 9 $$\sigma $$bonds. So now the pi bond in double bonded atoms is 1 that is the central C-C bond. The other 2 pi bonds in each triple bonded carbon nitrogen bonds are in total 8. So the total pi bonds are 9.
so the correct option for the question is option A.
Note: sigma bonds are more stronger than pi bonds because they hold the atoms rigidly in it. They also overlap the orbital directly. Due to which the electron density gets more concentrated between the two nuclei.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




