
Show how could you accomplish the following synthesis?
Cyclohexyl amine $\longrightarrow$ N- cyclohexyl acetamide
Answer
599.4k+ views
Hint: Cyclohexylamine is a cyclic, aromatic structure with 6 carbon and an amine group attached to it. For the conversion of amine to the amide group, the hydrogen is removed from the amine and carbonyl group (\[\text{C=O}\]) attaches in place of hydrogen and forms acetamide group i.e. \[\text{N}{{\text{H}}_{3}}\text{-CO-N}{{\text{H}}_{3}}\text{ }\] .
Complete answer:
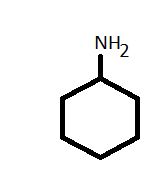
-To convert the cyclohexylamine let's draw the structure first.
-As it has 6- membered cyclic ring with an amine group so the structure is:
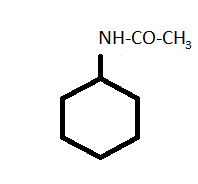
-Now, in N - cyclohexyl acetamide has a similar structure to cyclohexylamine except the presence of acetamide group in place of the amine group.
-For the synthesis of N - cyclohexyl acetamide from cyclohexylamine, pyridine is used as a catalyst to increase the rate or speed of the reaction.
-Along with the pyridine, acetyl chloride is used which has a molecular formula of $\text{C}{{\text{H}}_{3}}\text{-CO-Cl}$.
-From this, the oxidation of cyclohexylamine takes place due to which hydrogen atom releases from $\text{-N}{{\text{H}}_{2}}$ the group.
-Meanwhile, from acetyl chloride chlorine is released.
-So, the \[\text{C}{{\text{H}}_{3}}\text{ - CO-}\] group attaches with the amine group of the cyclohexylamine and it forms cyclohexyl acetamide.
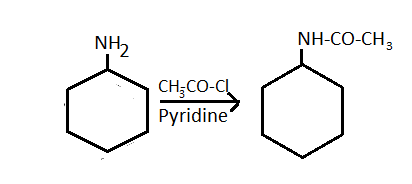
Cyclohexylamine N - cyclohexyl acetamide
-Along with it, hydrochloric acid is also formed.
-So, by using acetyl chloride we can synthesize N - cyclohexyl acetamide.
Note: It is observed that when N - cyclohexyl acetamide is reduced by $\text{LiAl}{{\text{H}}_{4}}$ which is a strong reducing agent, then the oxygen is lost from the acetamide and it can form N - ethyl cyclohexylamine, which has extra alkyl group than cyclohexylamine.
.
Complete answer:
-To convert the cyclohexylamine let's draw the structure first.
-As it has 6- membered cyclic ring with an amine group so the structure is:

-Now, in N - cyclohexyl acetamide has a similar structure to cyclohexylamine except the presence of acetamide group in place of the amine group.

-For the synthesis of N - cyclohexyl acetamide from cyclohexylamine, pyridine is used as a catalyst to increase the rate or speed of the reaction.
-Along with the pyridine, acetyl chloride is used which has a molecular formula of $\text{C}{{\text{H}}_{3}}\text{-CO-Cl}$.
-From this, the oxidation of cyclohexylamine takes place due to which hydrogen atom releases from $\text{-N}{{\text{H}}_{2}}$ the group.
-Meanwhile, from acetyl chloride chlorine is released.
-So, the \[\text{C}{{\text{H}}_{3}}\text{ - CO-}\] group attaches with the amine group of the cyclohexylamine and it forms cyclohexyl acetamide.

Cyclohexylamine N - cyclohexyl acetamide
-Along with it, hydrochloric acid is also formed.
-So, by using acetyl chloride we can synthesize N - cyclohexyl acetamide.
Note: It is observed that when N - cyclohexyl acetamide is reduced by $\text{LiAl}{{\text{H}}_{4}}$ which is a strong reducing agent, then the oxygen is lost from the acetamide and it can form N - ethyl cyclohexylamine, which has extra alkyl group than cyclohexylamine.
.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




