
How many secondary carbon atoms does methyl cyclopropane have?
Answer
590.7k+ views
Hint: To answer this question, firstly draw the structure of methyl cyclopropane. The structure will have a three membered ring with a methyl group attached to one carbon atom. The carbon atoms which are attached to two different carbon atoms will be the secondary carbon atom.
Complete answer:
Before answering this question, let us understand what secondary carbon atoms are.
There are three types of carbon atoms that may or may not be present in a compound.
The first is primary carbon atoms. The carbon atoms which are attached to only one more carbon atom is called a primary carbon. It is also referred to as ${{1}^{\circ }}$ carbon.
Next is the secondary carbon atom. The carbon atoms which are two different carbon atoms are called secondary carbon. It is also known as ${{2}^{\circ }}$ carbon.
Lastly, we have a tertiary carbon atom. The carbon atom which is bonded to three different carbon atoms is called a tertiary carbon. We also call it a ${{3}^{\circ }}$ carbon.
To find the number of secondary carbon atoms in methyl cyclopropane, firstly let us draw its structure.
As we can understand from the name, it will have a three membered cyclic ring structure with a methyl group attached to it. Therefore, we can draw its structure as-
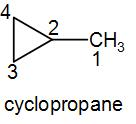
To understand it better, we have numbered the carbon atoms. Now let us discuss each carbon atom.
The carbon numbered as 1 is attached to only one more carbon atom (at 2) therefore this is a primary carbon atom.
The carbon numbered as 2 is attached to three different carbon atoms (1, 4 and 3) therefore this is a tertiary carbon atom.
The carbon atom numbered as 3 is attached to two different carbon atoms (2 and 4) therefore this is a secondary carbon atom.
Lastly, the carbon which we’ve numbered 4 is also attached to two different carbon atoms (3 and 2) therefore this is also a secondary carbon atom.
We can understand from the above discussion that there are two secondary carbon atoms.
Therefore, the correct answer is, there are two secondary carbon atoms in methyl cyclopropane.
Note:
A carbon atom can either be primary, secondary or tertiary and it can also be quaternary or ${{4}^{\circ }}$ carbon too. Such carbon would be attached to four other carbon atoms. Such carbon atoms are only found in hydrocarbons which have at least five carbon atoms. We can find quaternary carbons in branched chains but not in linear chains.
Complete answer:
Before answering this question, let us understand what secondary carbon atoms are.
There are three types of carbon atoms that may or may not be present in a compound.
The first is primary carbon atoms. The carbon atoms which are attached to only one more carbon atom is called a primary carbon. It is also referred to as ${{1}^{\circ }}$ carbon.
Next is the secondary carbon atom. The carbon atoms which are two different carbon atoms are called secondary carbon. It is also known as ${{2}^{\circ }}$ carbon.
Lastly, we have a tertiary carbon atom. The carbon atom which is bonded to three different carbon atoms is called a tertiary carbon. We also call it a ${{3}^{\circ }}$ carbon.
To find the number of secondary carbon atoms in methyl cyclopropane, firstly let us draw its structure.
As we can understand from the name, it will have a three membered cyclic ring structure with a methyl group attached to it. Therefore, we can draw its structure as-

To understand it better, we have numbered the carbon atoms. Now let us discuss each carbon atom.
The carbon numbered as 1 is attached to only one more carbon atom (at 2) therefore this is a primary carbon atom.
The carbon numbered as 2 is attached to three different carbon atoms (1, 4 and 3) therefore this is a tertiary carbon atom.
The carbon atom numbered as 3 is attached to two different carbon atoms (2 and 4) therefore this is a secondary carbon atom.
Lastly, the carbon which we’ve numbered 4 is also attached to two different carbon atoms (3 and 2) therefore this is also a secondary carbon atom.
We can understand from the above discussion that there are two secondary carbon atoms.
Therefore, the correct answer is, there are two secondary carbon atoms in methyl cyclopropane.
Note:
A carbon atom can either be primary, secondary or tertiary and it can also be quaternary or ${{4}^{\circ }}$ carbon too. Such carbon would be attached to four other carbon atoms. Such carbon atoms are only found in hydrocarbons which have at least five carbon atoms. We can find quaternary carbons in branched chains but not in linear chains.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




