
Salol prepared from:
A. Salicylic acid and methyl alcohol
B. Salicylic acid and phenol
C. Both 1 and 2
D. Aspirin and phenol
Answer
585k+ views
Hint: Salol is a benzoate ester and a member of salicylates and phenols. The chemical formula of salol is ${{\text{C}}_{13}}{{\text{H}}_{10}}{{\text{O}}_{3}}$. It is also used to manufacture polymers, waxes and polishes. Its IUPAC name is phenyl-2-hydroxybenzoate. Write the products formed by the reactants given in the options.
Complete step by step answer:
Let us discuss the products formed by the options:
A. Salicylic acid and methyl alcohol: Salicylic acid contains carboxylic acid, that’s why it is called as an acid. It also contains hydroxyl group at ortho position with respect to carboxylic acid group. It is mono-hydroxy benzoic acid. It has a chemical formula ${{\text{C}}_{7}}{{\text{H}}_{6}}{{\text{O}}_{3}}$.
Methyl alcohol contains hydroxyl groups attached to one carbon atom and rest valencies are filled by hydrogen atoms. It has a chemical formula $\text{C}{{\text{H}}_{3}}\text{OH}$.
The reaction of salicylic acid and methyl alcohol is an esterification reaction, which takes place in presence of ${{\text{H}}^{+}}$, which leads to formation of methyl salicylate or oil of wintergreen.
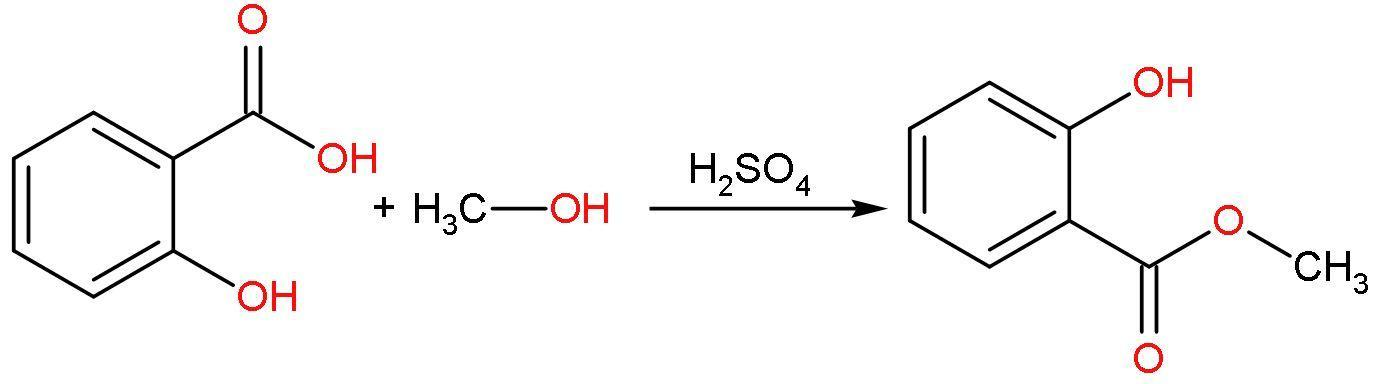
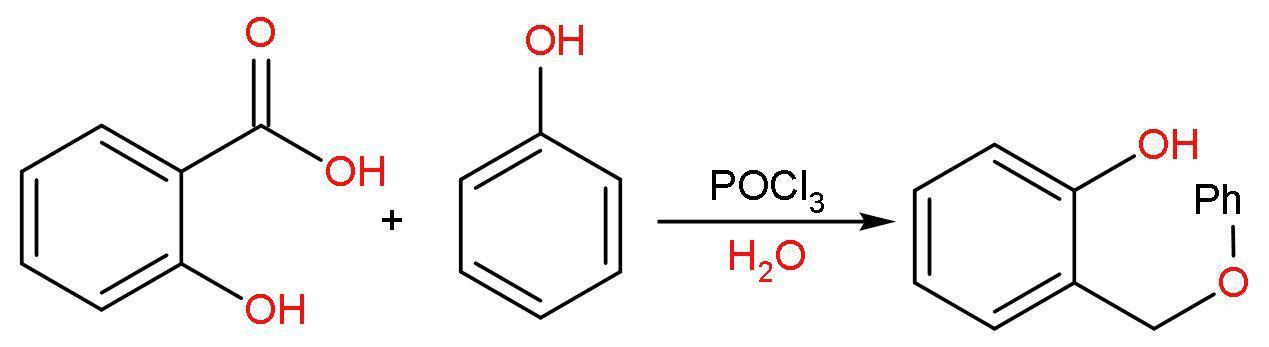
B. Salicylic acid and phenol: Salicylic acid contains carboxylic acid, that’s why it is called as an acid. It also contains hydroxyl group at ortho position with respect to carboxylic acid group. It is mono-hydroxy benzoic acid. It has a chemical formula ${{\text{C}}_{7}}{{\text{H}}_{6}}{{\text{O}}_{3}}$.
Phenol is an alcohol, in which hydroxyl group attached to benzene ring. The chemical formula of phenol is ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}$.
The reaction of salicylic acid and phenol is an esterification reaction, which takes place in presence of ${{\text{H}}^{+}}$, which leads to formation of phenol salicylate or salol.
D. Aspirin and phenol:
Aspirin is also known as acetylsalicylic acid. The IUPAC name of aspirin is 2-acetoxy benzoic acid. The chemical formula of aspirin is ${{\text{C}}_{9}}{{\text{H}}_{8}}{{\text{O}}_{4}}$.
Phenol is an alcohol, in which the hydroxyl group is attached to a benzene ring. The chemical formula of phenol is ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}$.
There is no reaction between aspirin and phenol.
Salol prepared from Salicylic acid and phenol, which is option ‘b’.
Additional Information: Uses of Salol are:
(1) It is used as an analgesic to relieve pain.
(2) It is used for treating inflammation in the lower urinary tract.
(3) It is used as an antiseptic with antipyretic and antibacterial effects for treatment of fever.
Note: Esterification reactions are reversible reactions. On reaction of carboxylic acids $\left( \text{RCOOH} \right)$ with alcohols $\left( \text{ROH} \right)$ in presence of acids like sulphuric acid $\left( {{\text{H}}_{2}}\text{S}{{\text{O}}_{4}} \right)$, gives the forward reaction of ester $\left( \text{RCOOR} \right)$ formation. If ester $\left( \text{RCOOR} \right)$ undergoes hydrolysis, then backward reaction is favoured. It gives back corresponding carboxylic acid $\left( \text{RCOOH} \right)$ and alcohol $\left( \text{ROH} \right)$.
Complete step by step answer:
Let us discuss the products formed by the options:
A. Salicylic acid and methyl alcohol: Salicylic acid contains carboxylic acid, that’s why it is called as an acid. It also contains hydroxyl group at ortho position with respect to carboxylic acid group. It is mono-hydroxy benzoic acid. It has a chemical formula ${{\text{C}}_{7}}{{\text{H}}_{6}}{{\text{O}}_{3}}$.
Methyl alcohol contains hydroxyl groups attached to one carbon atom and rest valencies are filled by hydrogen atoms. It has a chemical formula $\text{C}{{\text{H}}_{3}}\text{OH}$.
The reaction of salicylic acid and methyl alcohol is an esterification reaction, which takes place in presence of ${{\text{H}}^{+}}$, which leads to formation of methyl salicylate or oil of wintergreen.

B. Salicylic acid and phenol: Salicylic acid contains carboxylic acid, that’s why it is called as an acid. It also contains hydroxyl group at ortho position with respect to carboxylic acid group. It is mono-hydroxy benzoic acid. It has a chemical formula ${{\text{C}}_{7}}{{\text{H}}_{6}}{{\text{O}}_{3}}$.
Phenol is an alcohol, in which hydroxyl group attached to benzene ring. The chemical formula of phenol is ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}$.
The reaction of salicylic acid and phenol is an esterification reaction, which takes place in presence of ${{\text{H}}^{+}}$, which leads to formation of phenol salicylate or salol.

D. Aspirin and phenol:
Aspirin is also known as acetylsalicylic acid. The IUPAC name of aspirin is 2-acetoxy benzoic acid. The chemical formula of aspirin is ${{\text{C}}_{9}}{{\text{H}}_{8}}{{\text{O}}_{4}}$.
Phenol is an alcohol, in which the hydroxyl group is attached to a benzene ring. The chemical formula of phenol is ${{\text{C}}_{6}}{{\text{H}}_{5}}\text{OH}$.
There is no reaction between aspirin and phenol.
Salol prepared from Salicylic acid and phenol, which is option ‘b’.
Additional Information: Uses of Salol are:
(1) It is used as an analgesic to relieve pain.
(2) It is used for treating inflammation in the lower urinary tract.
(3) It is used as an antiseptic with antipyretic and antibacterial effects for treatment of fever.
Note: Esterification reactions are reversible reactions. On reaction of carboxylic acids $\left( \text{RCOOH} \right)$ with alcohols $\left( \text{ROH} \right)$ in presence of acids like sulphuric acid $\left( {{\text{H}}_{2}}\text{S}{{\text{O}}_{4}} \right)$, gives the forward reaction of ester $\left( \text{RCOOR} \right)$ formation. If ester $\left( \text{RCOOR} \right)$ undergoes hydrolysis, then backward reaction is favoured. It gives back corresponding carboxylic acid $\left( \text{RCOOH} \right)$ and alcohol $\left( \text{ROH} \right)$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




