
Relative stabilities of the following structures of are in this decreasing order:
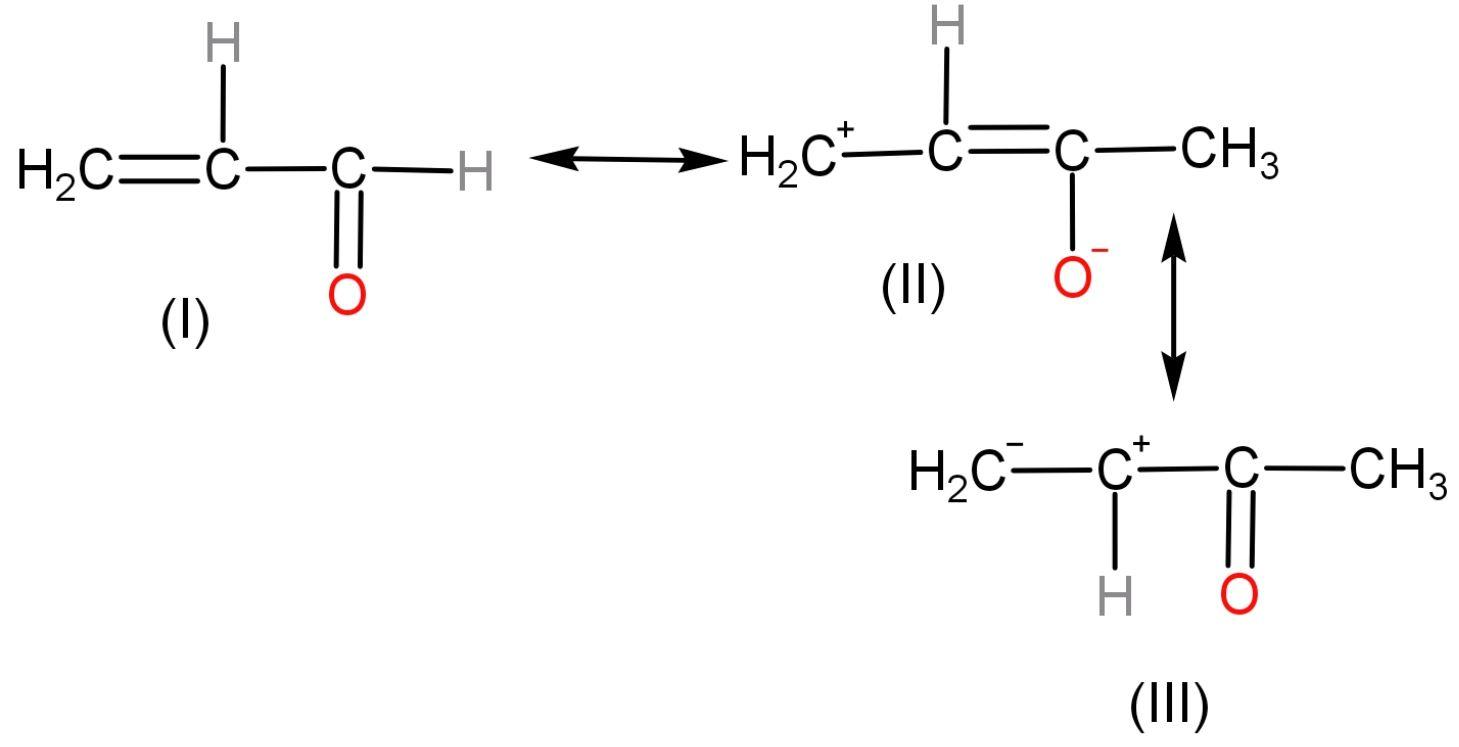
A. $\text{II}>\text{I}>\text{III}$
B. $\text{I}>\text{II}>\text{III}$
C. $\text{III}>\text{II}>\text{I}$
D. $\text{I}>\text{III}>\text{II}$

Answer
576.9k+ views
Hint:. The relative stabilities of the three compounds will be checked on the basis of the rules of stability. The rules include whether the compound with ions is stable more without ions. And which rule has more priority than another.
Complete step by step answer:
Let us see the rules first to judge the structures on the basis of stability. The rules are written in ascending order of priority.
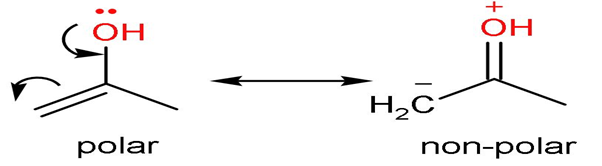
Rule (1)- Non-polar compounds are more stable than polar compounds. This is because covalent bonds are more stable and strong than ionic bonds.
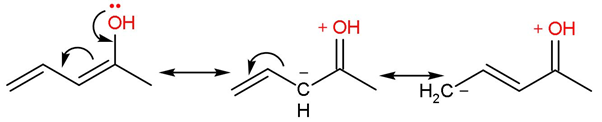
Rule (2)- The resonating structures where opposite charges are close are more stable and favourable because negative and positive are opposite charges on coming closer, they release a great amount of energy.
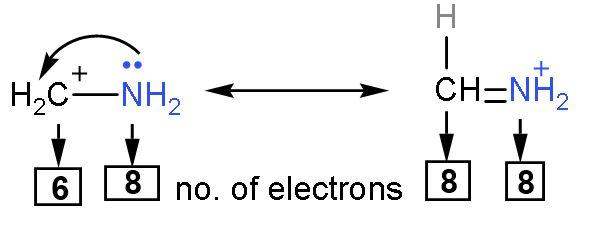
Rule (3)- The compound in which all elements have their octet complete are more stable. This is because elements achieve inert configuration by their octet completion.
Rule (4)- The resonating structures in which electronegative elements bear negative charge and electropositive elements bear positive charge are more favourable.
Let us now compare the three resonating structures given in the question rule-wise:
Hence, the conclusion of all the rules is that it is clear that compound I is most stable among other compounds as only it follows Rule (1). Then, among II and III, compound III is more stable as it follows rule (2) but compound II does not.
So, the correct answer is “Option D”.
Note: We while checking stability tend to forget rule (1) and rule (3) and their priorities we consider rule (4) prior rule (3). That’s where we make mistakes and the answer gets wrong. Remember that Rule (3) needs to be checked before checking Rule (4).
Complete step by step answer:
Let us see the rules first to judge the structures on the basis of stability. The rules are written in ascending order of priority.
Rule (1)- Non-polar compounds are more stable than polar compounds. This is because covalent bonds are more stable and strong than ionic bonds.

Rule (2)- The resonating structures where opposite charges are close are more stable and favourable because negative and positive are opposite charges on coming closer, they release a great amount of energy.

Rule (3)- The compound in which all elements have their octet complete are more stable. This is because elements achieve inert configuration by their octet completion.

Rule (4)- The resonating structures in which electronegative elements bear negative charge and electropositive elements bear positive charge are more favourable.

Let us now compare the three resonating structures given in the question rule-wise:
| Compounds | Structures | Rule 1 | Rule 2 | Rule 3 | Rule 4 |
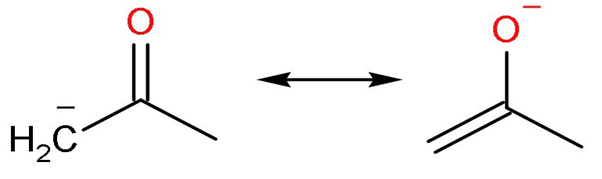
| I | 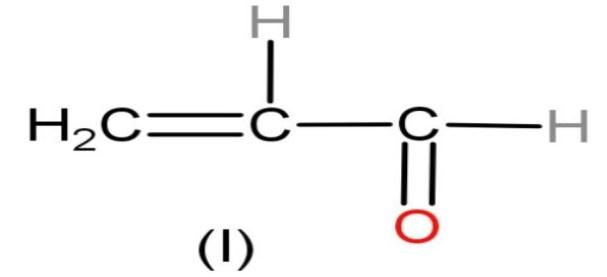
| Yes, the compound is nonpolar. | No charges are present | Octets of all the elements are complete. Carbon made four, oxygen made two and hydrogen made one bond. | No charges are present. |
| II | 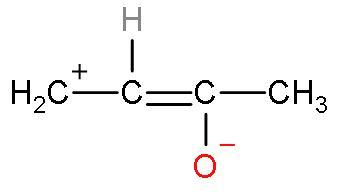
| The compound is polar. | Charges are present. The charges are present far away from each other. | Octets of all elements are complete except the first carbon atom from left. It has only 6 electrons. | The rule is followed as the more electronegative element ‘oxygen’ has negative charge and ‘electropositive’ element carbon has positive charge. |
| III | 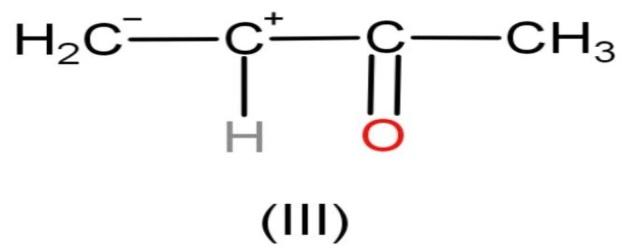
| The compound is polar. | Charges are present. The charges are very close to each other as they are present on adjacent carbons. | Octets of all elements are complete except the second carbon atom from left. It has only 6 electrons. | It follows this rule as the negative charge is present on less electronegative element. As, carbon is less electronegative than oxygen. |
Hence, the conclusion of all the rules is that it is clear that compound I is most stable among other compounds as only it follows Rule (1). Then, among II and III, compound III is more stable as it follows rule (2) but compound II does not.
So, the correct answer is “Option D”.
Note: We while checking stability tend to forget rule (1) and rule (3) and their priorities we consider rule (4) prior rule (3). That’s where we make mistakes and the answer gets wrong. Remember that Rule (3) needs to be checked before checking Rule (4).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




