
What is the reagent used in the Etard reaction?
A.Chromyl chloride
B.Ethanoyl chloride
C.Tin (II) chloride + hydrochloric acid
D.Cadmium chloride
Answer
600.9k+ views
Hint:
Etard reaction is a very important reaction, which is used for conversion of toluene to benzaldehyde. It is done by the oxidation of the alkyl group to an aldehyde group by an oxidizing reagent.
Complete step by step answer:
In the Etard reaction, we need a compound which has at least one methyl group bonded to a benzene ring. This methyl group is then oxidized to make an aldehyde. This is done by using a mild oxidant. Therefore, we use Chromyl chloride which is a chromium-based mild oxidizing agent.
The reaction is as follows –
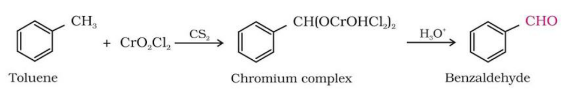
The reaction proceeds as the weak oxidizing agent reacts with toluene, which leads to the homolytic cleavage which leads to the formation of an intermediate known as an Etard complex. This complex is unstable. Therefore, on hydrolysis, there is a removal of two \[\text{Cr(OH}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\], which leads to the subsequent formation of benzaldehyde. This is also known as direct partial oxidation of toluene.
Therefore, the answer is – option (a) – Chromyl chloride.
Note:
The most common solvent used for this reaction is carbon tetrachloride. We can also use chloroform and carbon disulphide as a solvent. Taking non-polar solvent is important because it is inert and does not react with the reagent and the substrate.
Etard reaction is a very important reaction, which is used for conversion of toluene to benzaldehyde. It is done by the oxidation of the alkyl group to an aldehyde group by an oxidizing reagent.
Complete step by step answer:
In the Etard reaction, we need a compound which has at least one methyl group bonded to a benzene ring. This methyl group is then oxidized to make an aldehyde. This is done by using a mild oxidant. Therefore, we use Chromyl chloride which is a chromium-based mild oxidizing agent.
The reaction is as follows –

The reaction proceeds as the weak oxidizing agent reacts with toluene, which leads to the homolytic cleavage which leads to the formation of an intermediate known as an Etard complex. This complex is unstable. Therefore, on hydrolysis, there is a removal of two \[\text{Cr(OH}{{\text{)}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\], which leads to the subsequent formation of benzaldehyde. This is also known as direct partial oxidation of toluene.
Therefore, the answer is – option (a) – Chromyl chloride.
Note:
The most common solvent used for this reaction is carbon tetrachloride. We can also use chloroform and carbon disulphide as a solvent. Taking non-polar solvent is important because it is inert and does not react with the reagent and the substrate.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




