
Ratio of $s{{p}^{3}}$ and $s{{p}^{2}}$ hybridized atoms in the anionic part of the borax is: (if answer is 12:4, then represent as 124)
(a)- 0101
(b)- 1201
(c)- 1121
(d)- 1321
Answer
565.5k+ views
Hint: The formula of borax is $N{{a}_{2}}[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}].8{{H}_{2}}O$, but in simplified form it is written as $N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O$. So the anionic part of borax has formula ${{[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}]}^{2-}}$. The hybridization can be calculated by the structure of the specific boron atom.
Complete answer:
Borax is a compound of boron element of group 13 in which sodium is also present. The formula of borax is $N{{a}_{2}}[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}].8{{H}_{2}}O$, but in simplified form it is written as $N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O$. So the anionic part of borax has formula ${{[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}]}^{2-}}$. It is a very important compound that is used for the preparation of tiles, pottery, etc.
Actually, the structure of the anionic form of borax contains tetranuclear units. So in the structure, two boron atoms have a negative charge and two boron atoms are neutral, each boron atom has one hydroxyl group and all the boron atoms are joined together with oxygen bonds. The structure is given below:
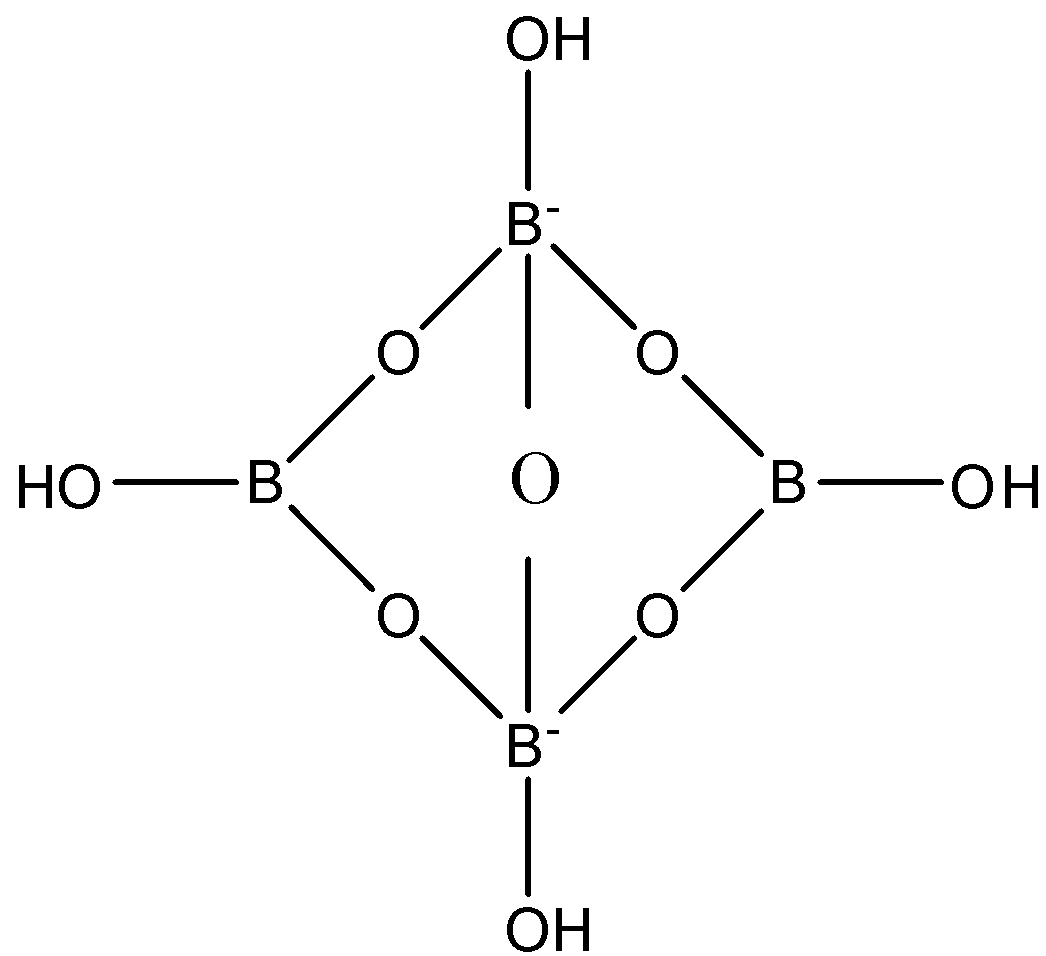
Sometimes, we cannot predict the hybridization of the atom by the number of bonds, so we have to see the geometry of the atom. So in the anionic part of borax, the two boron atoms that have negative charge have tetrahedral geometry and the two neutral boron atoms have trigonal planar geometry. And we know that the tetrahedral geometry have $s{{p}^{3}}$hybridization and trigonal planar have $s{{p}^{2}}$hybridization.
So their ratio is 2:2, which can be simplified as 1:1.
Therefore, the correct answer is option (a)- 0101.
Note:
If the geometry is linear, then the hybridization is $sp$. Borax has antiseptic property so it is used in medicinal soaps and laboratories and it is used for borax bead test.
Complete answer:
Borax is a compound of boron element of group 13 in which sodium is also present. The formula of borax is $N{{a}_{2}}[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}].8{{H}_{2}}O$, but in simplified form it is written as $N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O$. So the anionic part of borax has formula ${{[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}]}^{2-}}$. It is a very important compound that is used for the preparation of tiles, pottery, etc.
Actually, the structure of the anionic form of borax contains tetranuclear units. So in the structure, two boron atoms have a negative charge and two boron atoms are neutral, each boron atom has one hydroxyl group and all the boron atoms are joined together with oxygen bonds. The structure is given below:

Sometimes, we cannot predict the hybridization of the atom by the number of bonds, so we have to see the geometry of the atom. So in the anionic part of borax, the two boron atoms that have negative charge have tetrahedral geometry and the two neutral boron atoms have trigonal planar geometry. And we know that the tetrahedral geometry have $s{{p}^{3}}$hybridization and trigonal planar have $s{{p}^{2}}$hybridization.
So their ratio is 2:2, which can be simplified as 1:1.
Therefore, the correct answer is option (a)- 0101.
Note:
If the geometry is linear, then the hybridization is $sp$. Borax has antiseptic property so it is used in medicinal soaps and laboratories and it is used for borax bead test.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




