
Propane can be best prepared by the reaction:
A. $C{H_3}C{H_2}I + C{H_3}I + Na\xrightarrow{{E{t_2}O}}$
B. $C{H_3}C{H_2}COONa + C{H_3}COONa\xrightarrow[{Electrolysis}]{{{H_2}O}}$
C. $C{H_3}C{H_2}Br + {(C{H_3})_2}CuLi\xrightarrow{{E{t_2}O}}$
D. $C{H_3}C{H_2}C{H_2}COONa\xrightarrow[{CuO,\Delta }]{{NaOH}}$
Answer
554.1k+ views
Hint: Propane can be prepared by wurtz reaction, in which sodium as a reactant is present along with the haloalkane with the same number of carbon atoms as desired in the product, which is three in case of propane.
With the help of wurtz reaction we can also make unsymmetric hydrocarbons by taking both the haloalkanes of different numbers of carbon atoms.
Complete step by step answer:
The Wurtz Coupling reaction is one of the oldest organic reactions, and this reaction produces the simple dimer which is derived from two equivalent molecules of alkyl halide. They are also used in preparation of strained ring compounds because of their intramolecular version of the reaction. For instance, given below is an example of formation of a cyclic compound by using wurtz reaction. Here we can see that the reactant is cyclic in nature with two halogen functional groups attached to it. These halogens will react with the sodium with Is also present as a reactant, and the two free sites are bound with each other by a single bond, in the product.
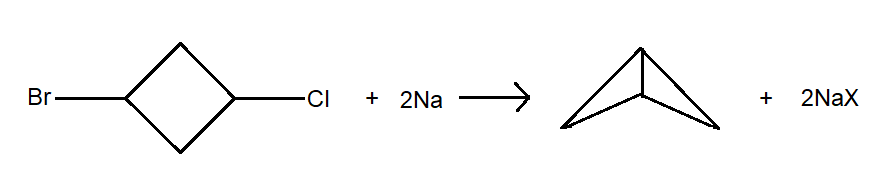
Now we will look at the general mechanism which takes place during a wurtz reaction.
$R - X + 2Na \to {R^ - }N{a^ + } + NaX$
${R^ - }N{a^ + } + R - X \to R - R + NaX$
As we can see this reaction is a two step process. In the first step the haloalkane reacts with two molecules of sodium in order to form an intermediate of the hydrocarbon part along with the sodium ion, and a part of salt is formed as a side product. The nature of salt depends on which halogen we are taking. If we take chlorine as the halogen then sodium chloride as a salt will be formed. Similarly, if we take bromine as the halogen then sodium bromide as a salt will be formed.
In the next step as we can see this intermediate reacts with another molecule of haloalkane, in order to form a long chain of hydrocarbons which will contain both the hydrocarbon parts of the reactant. And in the same way, sodium salt of the halogen will be formed as a side product.
Now as you can see in the first option, reactants are haloalkanes and sodium is also present as a reactant. So if we combine both the hydrocarbons involved in the reactant, propane will be formed.
$C{H_3}C{H_2}I + C{H_3}I + Na\xrightarrow{{{H_2}O}}C{H_3}C{H_2}C{H_3} + NaI$
So, the correct answer is Option A.
Note: Propane is produced as a by-product of two other processes, natural gas processing and petroleum refining.
Propane combustion, that is reaction of propane with oxygen is much cleaner than that of coal or unleaded gasoline.
With the help of wurtz reaction we can also make unsymmetric hydrocarbons by taking both the haloalkanes of different numbers of carbon atoms.
Complete step by step answer:
The Wurtz Coupling reaction is one of the oldest organic reactions, and this reaction produces the simple dimer which is derived from two equivalent molecules of alkyl halide. They are also used in preparation of strained ring compounds because of their intramolecular version of the reaction. For instance, given below is an example of formation of a cyclic compound by using wurtz reaction. Here we can see that the reactant is cyclic in nature with two halogen functional groups attached to it. These halogens will react with the sodium with Is also present as a reactant, and the two free sites are bound with each other by a single bond, in the product.
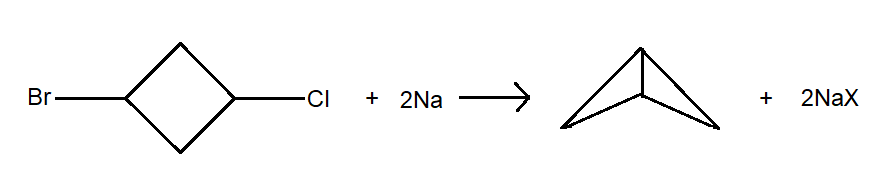
Now we will look at the general mechanism which takes place during a wurtz reaction.
$R - X + 2Na \to {R^ - }N{a^ + } + NaX$
${R^ - }N{a^ + } + R - X \to R - R + NaX$
As we can see this reaction is a two step process. In the first step the haloalkane reacts with two molecules of sodium in order to form an intermediate of the hydrocarbon part along with the sodium ion, and a part of salt is formed as a side product. The nature of salt depends on which halogen we are taking. If we take chlorine as the halogen then sodium chloride as a salt will be formed. Similarly, if we take bromine as the halogen then sodium bromide as a salt will be formed.
In the next step as we can see this intermediate reacts with another molecule of haloalkane, in order to form a long chain of hydrocarbons which will contain both the hydrocarbon parts of the reactant. And in the same way, sodium salt of the halogen will be formed as a side product.
Now as you can see in the first option, reactants are haloalkanes and sodium is also present as a reactant. So if we combine both the hydrocarbons involved in the reactant, propane will be formed.
$C{H_3}C{H_2}I + C{H_3}I + Na\xrightarrow{{{H_2}O}}C{H_3}C{H_2}C{H_3} + NaI$
So, the correct answer is Option A.
Note: Propane is produced as a by-product of two other processes, natural gas processing and petroleum refining.
Propane combustion, that is reaction of propane with oxygen is much cleaner than that of coal or unleaded gasoline.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




