
What is the product (Q) of the following reaction?
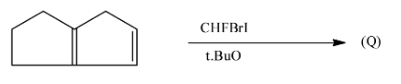
A.
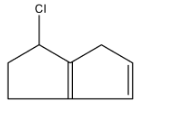
B.
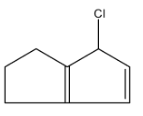
C.
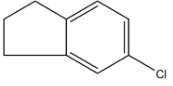
D.
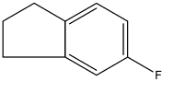





Answer
534.9k+ views
Hint: When CHFBrI will react with tertiary butoxide then, there is the formation of carbene, this carbine will react with the reactant molecule and insert itself in the reactant and then there will be the formation of carbocation leaving the most electronegative on the ring.
Complete step by step answer: Let us first see, what is the reaction between the molecules written on the arrow in the reaction, so, there are two compounds written, i.e., CHFBrI and t-BuO. CHFBrI is Bromochloroiodomethane and t-BuO is tertiary butoxide, so these react together, then there will be the formation of carbene in which the carbon atom will have a lone pair, fluorine and bromine atom. The reaction is given below:
$CHFBrI\xrightarrow{t-Bu{{O}^{-}}}\overset{-}{\mathop{C}}\,FBrI\xrightarrow{{{I}^{-}}}:CFBr$
This carbene will attack the reactant. The given reactant is a fused two pentene rings, so the carbine will attack one of the double bonds and will form a six-membered ring. After the formation of a six-membered ring, the bromine will leave and there will be the formation of a carbocation. This carbocation will be balanced by the formation of a double bond by the removal of a hydrogen atom from the ring. The series of reaction is given below:
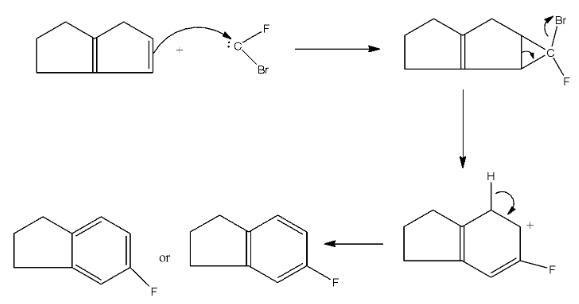
So, the obtained product is equal to the compounds given in option (d). Therefore, the correct answer is an option (d).
Note: In the formation of carbene, the iodine was removed because the electronegativity of iodine is less than the electronegativity of bromine and fluorine. So, the atom that has the highest electronegativity will retain the aromatic ring.
Complete step by step answer: Let us first see, what is the reaction between the molecules written on the arrow in the reaction, so, there are two compounds written, i.e., CHFBrI and t-BuO. CHFBrI is Bromochloroiodomethane and t-BuO is tertiary butoxide, so these react together, then there will be the formation of carbene in which the carbon atom will have a lone pair, fluorine and bromine atom. The reaction is given below:
$CHFBrI\xrightarrow{t-Bu{{O}^{-}}}\overset{-}{\mathop{C}}\,FBrI\xrightarrow{{{I}^{-}}}:CFBr$
This carbene will attack the reactant. The given reactant is a fused two pentene rings, so the carbine will attack one of the double bonds and will form a six-membered ring. After the formation of a six-membered ring, the bromine will leave and there will be the formation of a carbocation. This carbocation will be balanced by the formation of a double bond by the removal of a hydrogen atom from the ring. The series of reaction is given below:

So, the obtained product is equal to the compounds given in option (d). Therefore, the correct answer is an option (d).
Note: In the formation of carbene, the iodine was removed because the electronegativity of iodine is less than the electronegativity of bromine and fluorine. So, the atom that has the highest electronegativity will retain the aromatic ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




