
What is the product of the Simmons-Smith reaction with cyclopentene?
a.) Bicyclo (3.1.0)-pentane
b.) Bicyclo (3.1.0)-hexane
c.) Bicyclo (5.3.0)-hexane
d.) Bicyclo (5.3.0)-pentane
Answer
568.5k+ views
Hint: In Simmons-smith reaction cycloalkene reacts with diiodomethane ($C{{H}_{2}}{{I}_{2}}$) in presence of metallic zinc and copper to form a Bicyclo compound where a carbenoid gets added on pi-bond to form a bridge that contains one carbon atom.
Complete step by step answer:
- The iodomethyl zinc iodide is usually prepared using Zn activated with Cu.
The iodomethyl zinc iodide reacts with a cycloalkene to give a bicyclo compound.
\[C{{H}_{2}}{{I}_{2}}+Zn\to IZnC{{H}_{2}}I\]
- The iodomethyl zinc iodide reacts with a cycloalkene to give a bicyclo compound.
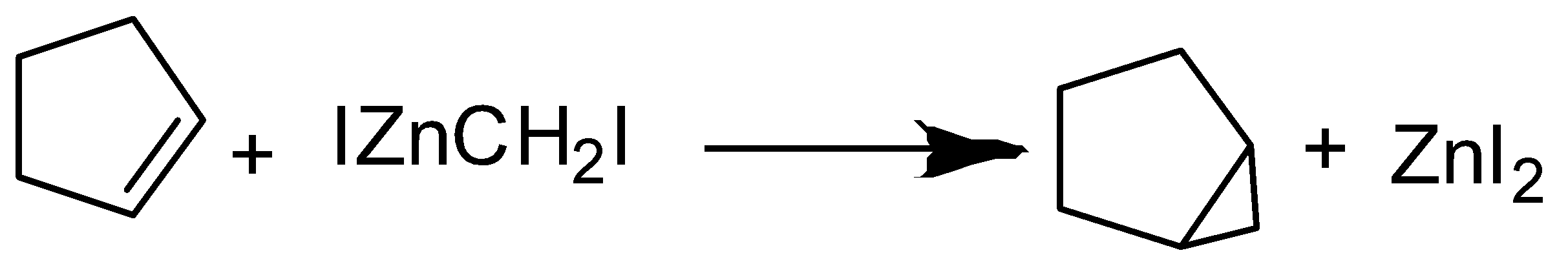
Nomenclature of Bicyclo compounds:
- We name compounds containing two fused or bridged rings as bicyclo alkanes. Use the name of the alkane corresponding to the total number of carbon atoms in the rings as the parent name. Our bicyclo compound has a total 6 carbon atoms So, it will be hexane.
- The carbon atoms common to both rings are called bridgeheads, and each bond, or each chain of atoms, connecting the bridgehead atoms is called a bridge. Use brackets to denote the number of carbon atoms in each bridge (in order of decreasing length).
- In our bicyclo compound there are three bridges where one bridge contains 4 carbon atoms, the second bridge that contains 1 carbon atom and another one that connects both the bridges doesn’t contain any carbon atom other than two bridgehead carbon atoms. So, the order will be 3,1,0.
- If substituents are present, number the bridged ring system beginning at one bridgehead, proceeding first along the longest bridge to the other bridgehead, then along the next longest bridge back to the first bridgehead. The shortest bridge is numbered last. As no substituent is present So, this step will not be required.
So, name of our bicyclo compound will be:
Bicyclo [3,1,0] hexane
The correct answer is option “B” .
Additional Information :The Simmons-Smith reaction can be used for both straight-chained and closed-chained alkene. For example, both butene and cyclohexene will undergo this reaction.
Note: The reaction is stereospecific to the alkene. For example, if the alkyl groups of the alkene are cis- then they are also, cis- in the cyclopropane and trans-alkenes. The intermediates in the Simmons–Smith reaction show much more controlled reactivity than, for example, diazoalkanes. Competing side reactions are greatly reduced.
Complete step by step answer:
- The iodomethyl zinc iodide is usually prepared using Zn activated with Cu.
The iodomethyl zinc iodide reacts with a cycloalkene to give a bicyclo compound.
\[C{{H}_{2}}{{I}_{2}}+Zn\to IZnC{{H}_{2}}I\]
- The iodomethyl zinc iodide reacts with a cycloalkene to give a bicyclo compound.
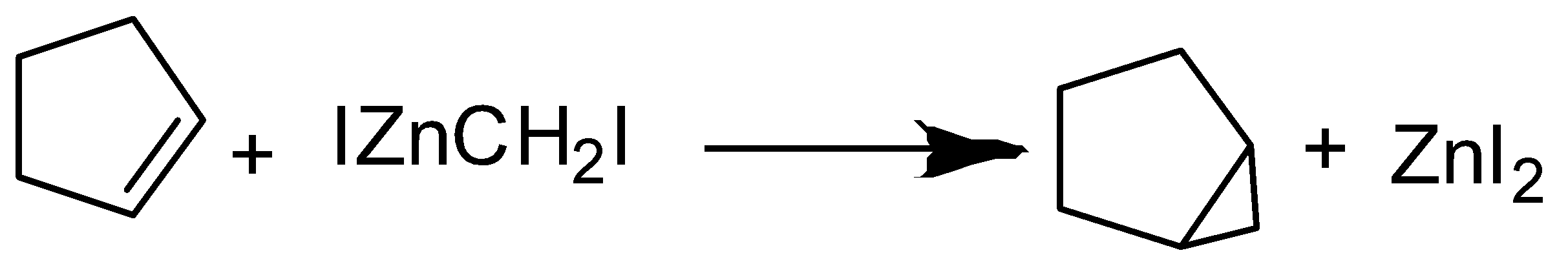
Nomenclature of Bicyclo compounds:
- We name compounds containing two fused or bridged rings as bicyclo alkanes. Use the name of the alkane corresponding to the total number of carbon atoms in the rings as the parent name. Our bicyclo compound has a total 6 carbon atoms So, it will be hexane.
- The carbon atoms common to both rings are called bridgeheads, and each bond, or each chain of atoms, connecting the bridgehead atoms is called a bridge. Use brackets to denote the number of carbon atoms in each bridge (in order of decreasing length).
- In our bicyclo compound there are three bridges where one bridge contains 4 carbon atoms, the second bridge that contains 1 carbon atom and another one that connects both the bridges doesn’t contain any carbon atom other than two bridgehead carbon atoms. So, the order will be 3,1,0.
- If substituents are present, number the bridged ring system beginning at one bridgehead, proceeding first along the longest bridge to the other bridgehead, then along the next longest bridge back to the first bridgehead. The shortest bridge is numbered last. As no substituent is present So, this step will not be required.
So, name of our bicyclo compound will be:
Bicyclo [3,1,0] hexane
The correct answer is option “B” .
Additional Information :The Simmons-Smith reaction can be used for both straight-chained and closed-chained alkene. For example, both butene and cyclohexene will undergo this reaction.
Note: The reaction is stereospecific to the alkene. For example, if the alkyl groups of the alkene are cis- then they are also, cis- in the cyclopropane and trans-alkenes. The intermediates in the Simmons–Smith reaction show much more controlled reactivity than, for example, diazoalkanes. Competing side reactions are greatly reduced.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




