
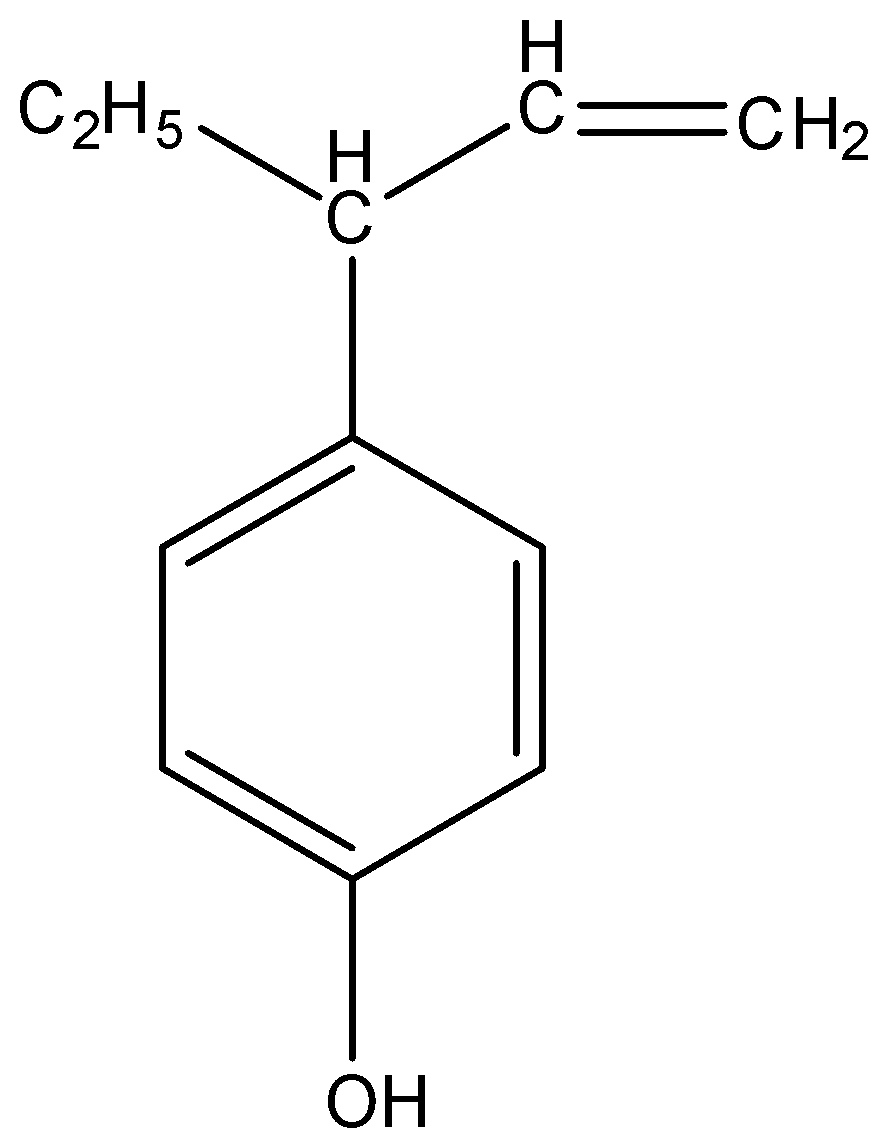
What is the product obtained by heating the following allylic ether of phenol?
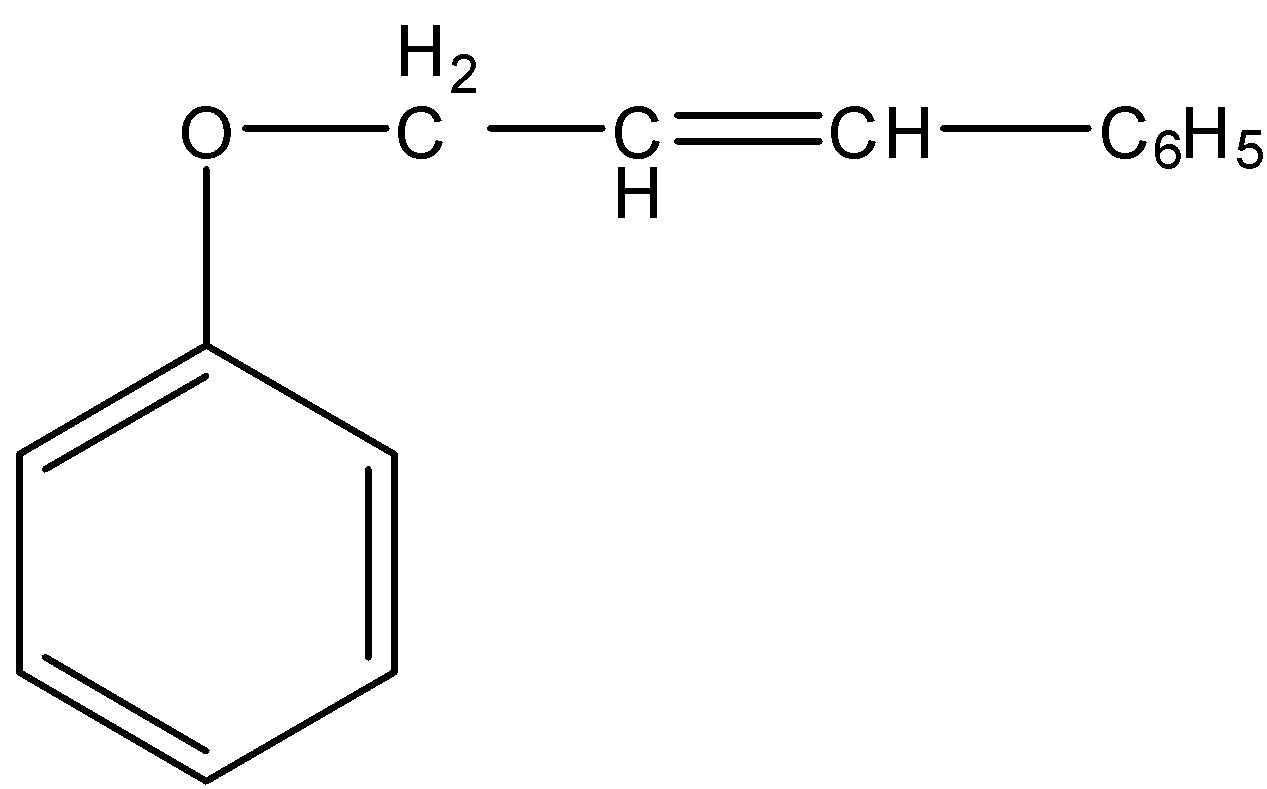
(A)
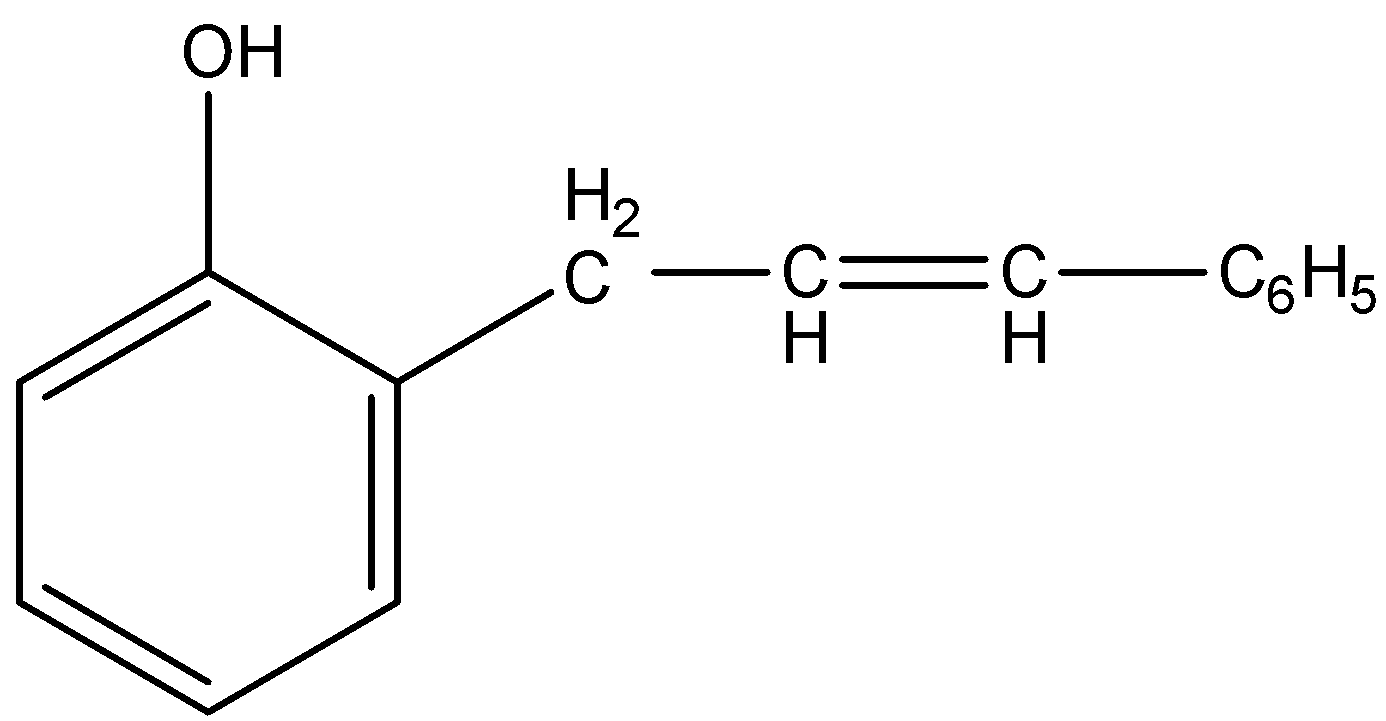
(B)
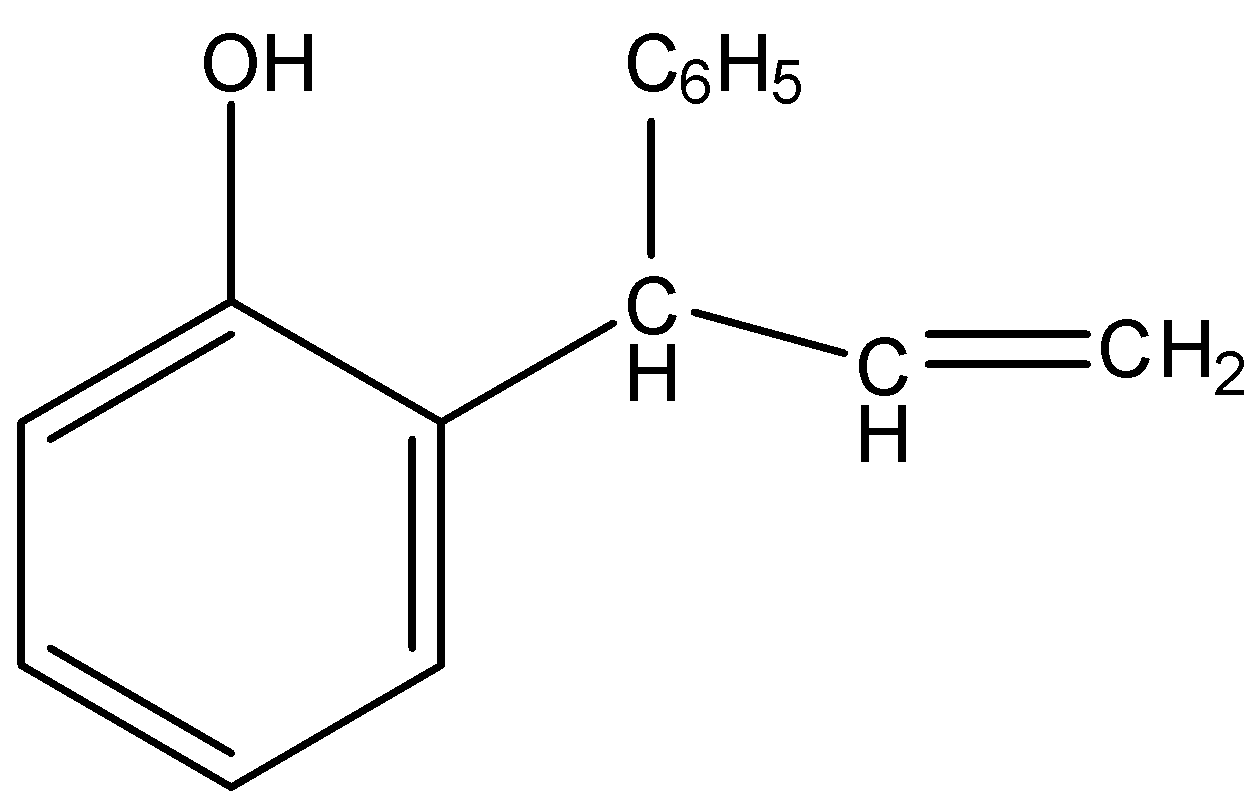
(C)
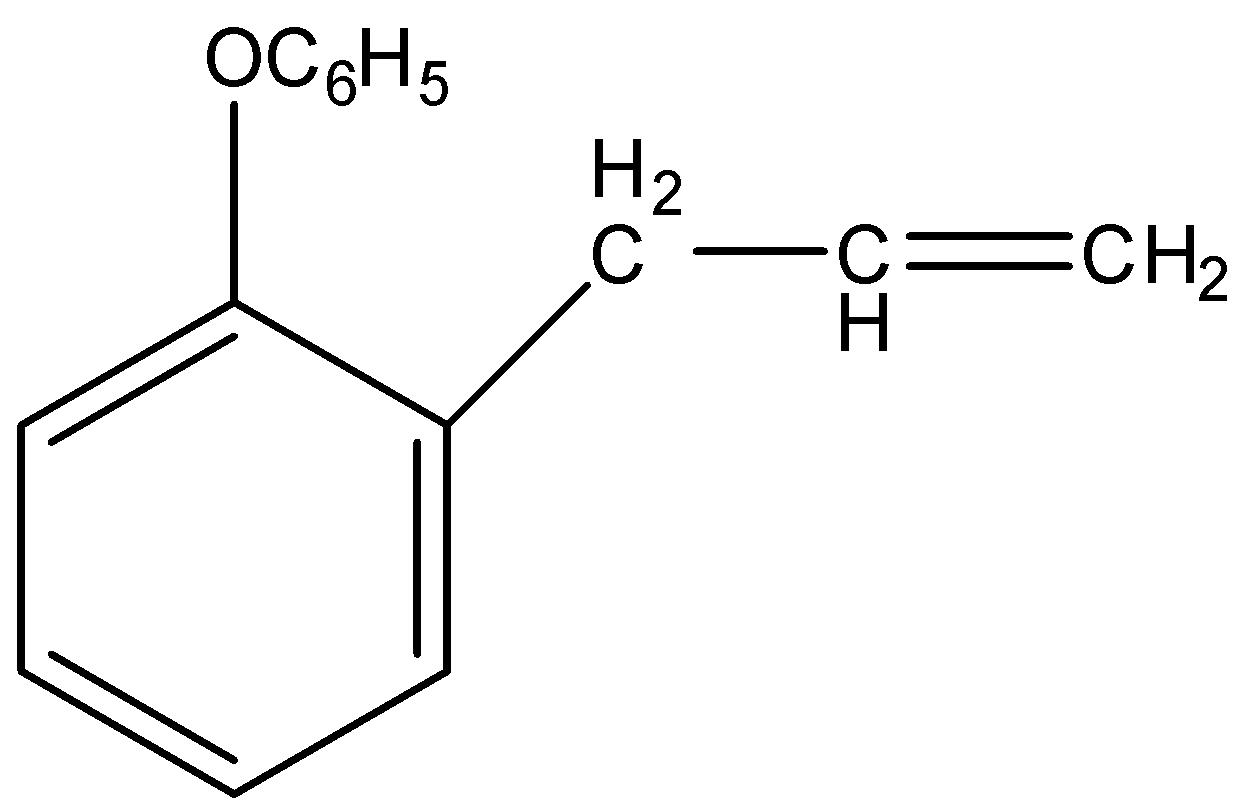
(D)





Answer
570k+ views
Hint: We should understand the nature of bonds in the allyl phenol ether. On heating which bond will be most readily broken will be predicted by the nature of bond. There is more probability of rearrangement in these types of compounds.
Complete step by step answer:
Rearrangements are very common in allylic or vinylic ether. The heating of allylic ether will only start a ${{[3,3] sigmatropic}}$ rearrangement will occur to give ${{\gamma ,\delta - unsaturated}}$ carbonyl compound. This is called the Claisen rearrangement.
Here is the mechanism followed:
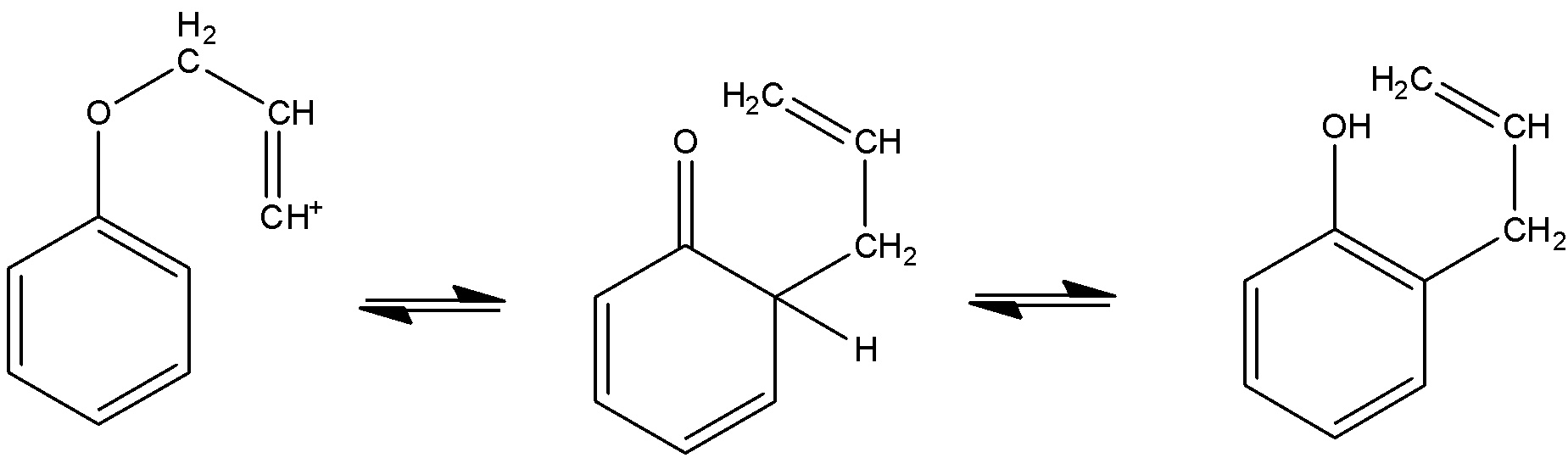
The heating of allylic phenol ether will also follow the same mechanism given above
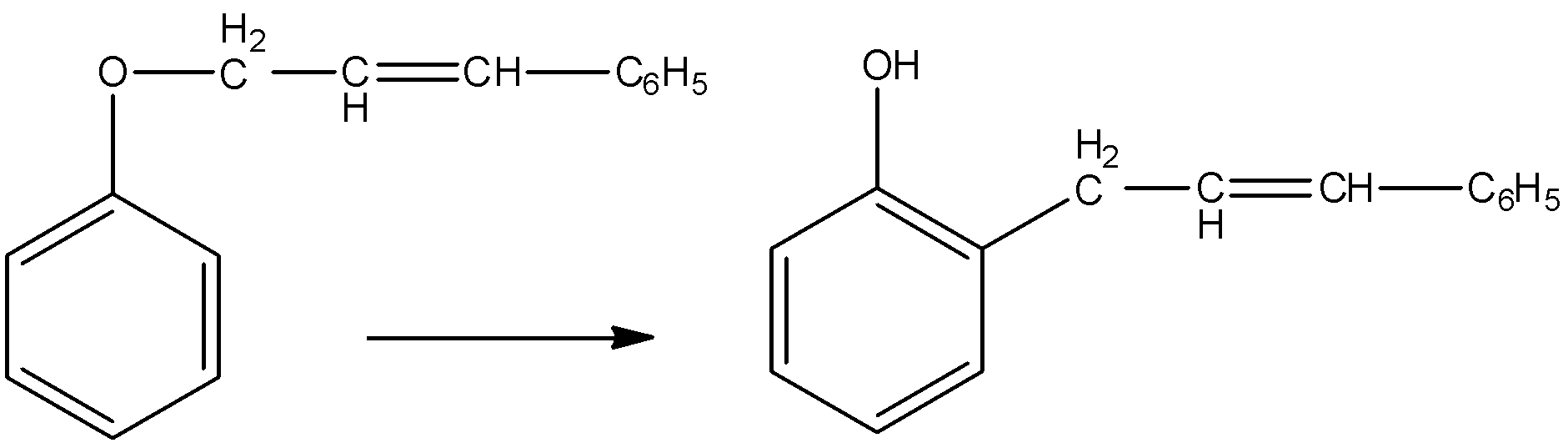
This is a Claisen rearrangement and happens through a ${{[3,3]\; sigmatropic}}$ arrangement. The Claisen rearrangement also happens in aliphatic substrate. In this aliphatic rearrangement a [3,3]-sigma tropic rearrangement in an allyl vinyl ether will be converted thermally to an unsaturated carbonyl compound.
The aromatic Claisen Rearrangement also happens and it is accompanied by a rearomatization.
So, the correct answer is “Option A”.
Additional information:
The name of Claisen rearrangement is named after Rainer Ludwig Claisen. These reactions are classified in sigma tropic rearrangements. These are concerted processes in which the bonds are breaking and forming at the same time in the reaction.
Note: When substituents are present as an allylic ether the stereochemistry is very important in this reaction. Stereochemistry is very important for the product. It can be predicted by drawing the correct structure of the starting material in chair form.
Complete step by step answer:
Rearrangements are very common in allylic or vinylic ether. The heating of allylic ether will only start a ${{[3,3] sigmatropic}}$ rearrangement will occur to give ${{\gamma ,\delta - unsaturated}}$ carbonyl compound. This is called the Claisen rearrangement.
Here is the mechanism followed:

The heating of allylic phenol ether will also follow the same mechanism given above

This is a Claisen rearrangement and happens through a ${{[3,3]\; sigmatropic}}$ arrangement. The Claisen rearrangement also happens in aliphatic substrate. In this aliphatic rearrangement a [3,3]-sigma tropic rearrangement in an allyl vinyl ether will be converted thermally to an unsaturated carbonyl compound.
The aromatic Claisen Rearrangement also happens and it is accompanied by a rearomatization.
So, the correct answer is “Option A”.
Additional information:
The name of Claisen rearrangement is named after Rainer Ludwig Claisen. These reactions are classified in sigma tropic rearrangements. These are concerted processes in which the bonds are breaking and forming at the same time in the reaction.
Note: When substituents are present as an allylic ether the stereochemistry is very important in this reaction. Stereochemistry is very important for the product. It can be predicted by drawing the correct structure of the starting material in chair form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




