
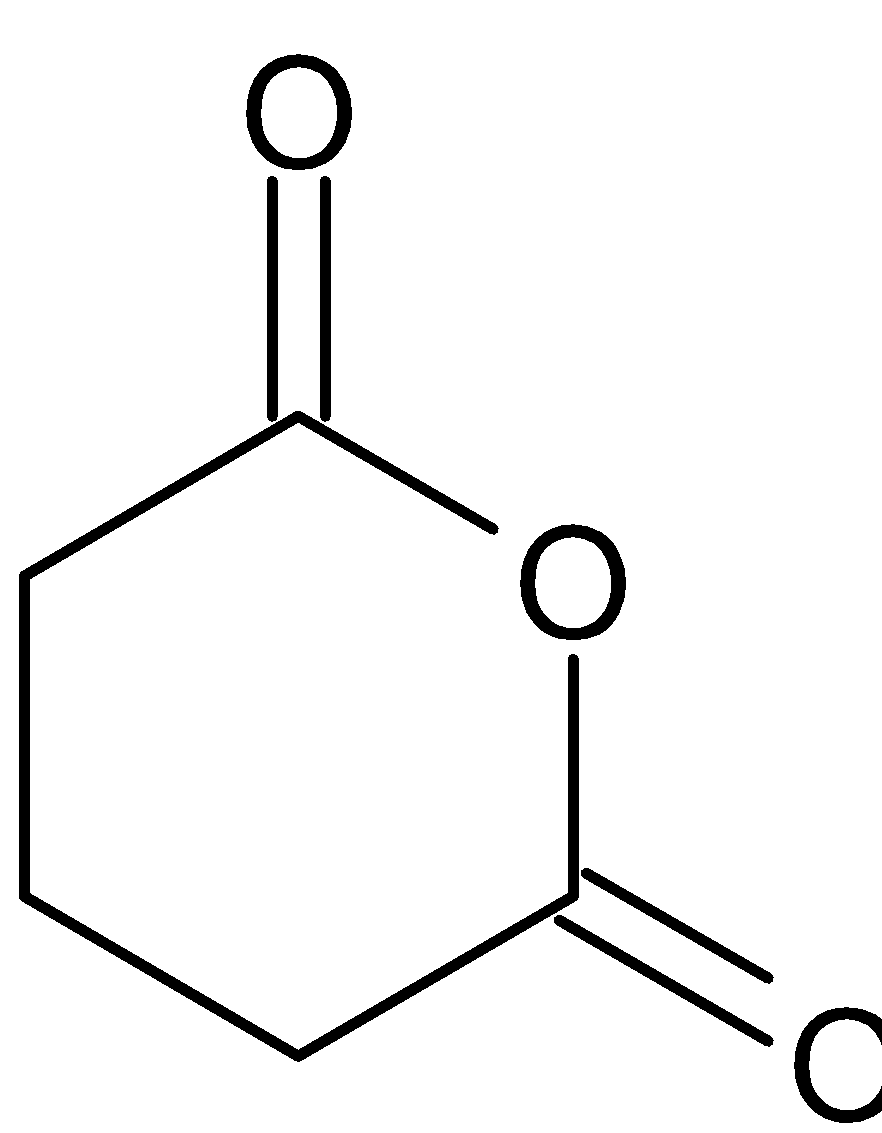
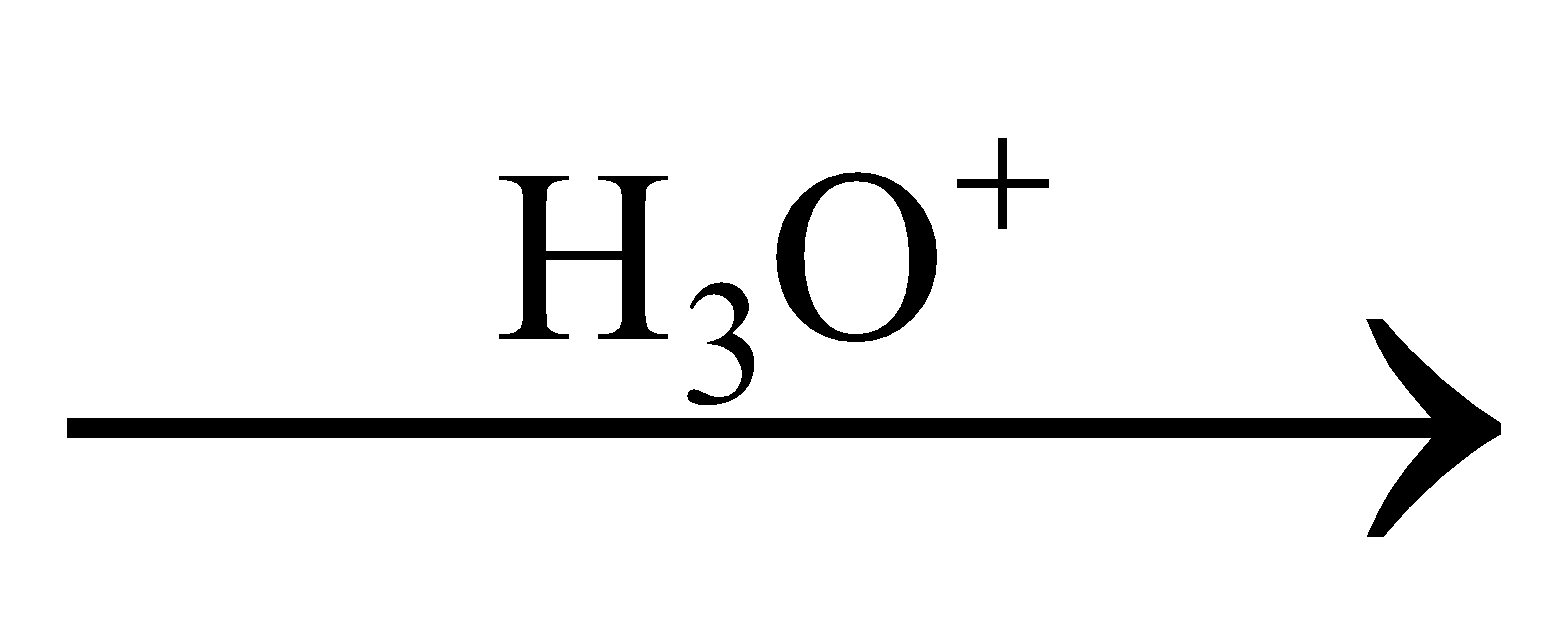 Product is:
Product is:
1. Succinic acid
2. Malonic acid
3. Adipic acid
4. Glutaric acid

Answer
558.3k+ views
Hint: Glutaric anhydride is also known as tetrahydropyran-2,6-dione, here the Carbonyl group is susceptible for nucleophilic attack
This reaction is a simple acid-base reaction.
Complete step by step answer:
Carboxylic acids on heating with certain dehydrating agents like Sulphuric acid undergoes elimination of water giving corresponding anhydrides
The Glutaric anhydride formed is hydrolysed with water forming Glutaric acids
Here the lone pair of electrons present on Oxygen attacks the partially positive Carbon of Carbonyl group resulting in the formation of acids
The complete reaction is given by:
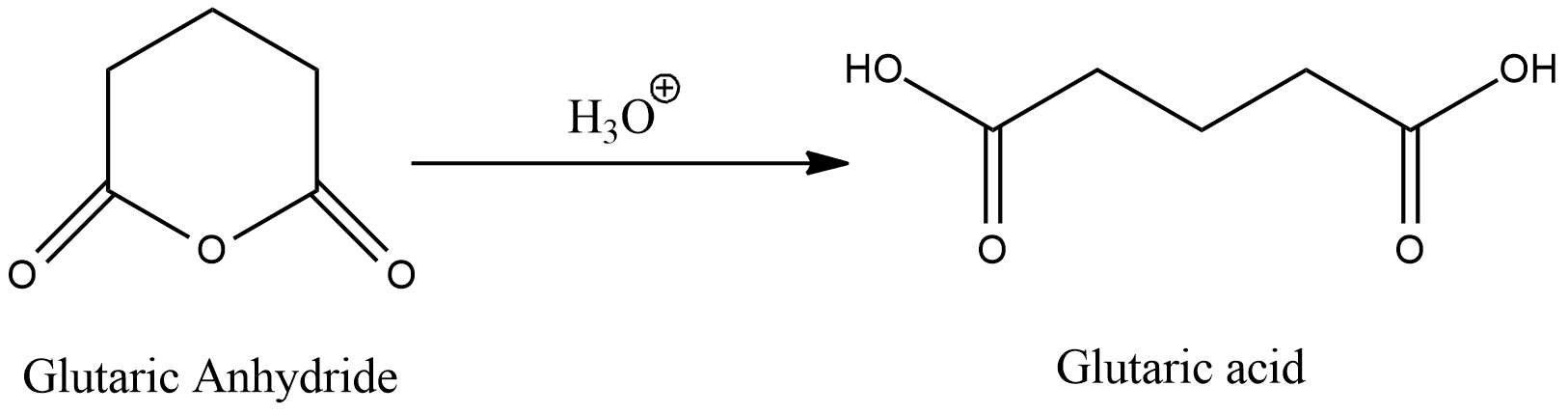
Therefore, the product of this reaction is Glutaric acid.
The correct answer is (4).
Additional Information:
Glutaric acid has a molecular formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\left( {{\text{COOH}}} \right)_{\text{2}}}$ . It is a water-soluble compound with solubility up to fifty percent.
Glutaric acid has got a Molecular weight of 132.12 ${\text{g/mol}}$ , Melting point of 95 to 98 $^{\text{o}}{\text{C}}$ and Boiling points of 200 $^{\text{o}}{\text{C}}$
It is found in variety of food items such as beetroot, oat
It is produced in our body naturally during the metabolic activity of amino acid.
Note:
Glutaric acid finds its applications in manufacture of polymers such as polyamides and because of the presence of an odd number of carbon atoms it is useful in decreasing polymer elasticity. It can cause eye and skin irritation when comes in contact.
This reaction is a simple acid-base reaction.
Complete step by step answer:
Carboxylic acids on heating with certain dehydrating agents like Sulphuric acid undergoes elimination of water giving corresponding anhydrides
The Glutaric anhydride formed is hydrolysed with water forming Glutaric acids
Here the lone pair of electrons present on Oxygen attacks the partially positive Carbon of Carbonyl group resulting in the formation of acids
The complete reaction is given by:

Therefore, the product of this reaction is Glutaric acid.
The correct answer is (4).
Additional Information:
Glutaric acid has a molecular formula ${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\left( {{\text{COOH}}} \right)_{\text{2}}}$ . It is a water-soluble compound with solubility up to fifty percent.
Glutaric acid has got a Molecular weight of 132.12 ${\text{g/mol}}$ , Melting point of 95 to 98 $^{\text{o}}{\text{C}}$ and Boiling points of 200 $^{\text{o}}{\text{C}}$
It is found in variety of food items such as beetroot, oat
It is produced in our body naturally during the metabolic activity of amino acid.
Note:
Glutaric acid finds its applications in manufacture of polymers such as polyamides and because of the presence of an odd number of carbon atoms it is useful in decreasing polymer elasticity. It can cause eye and skin irritation when comes in contact.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




