
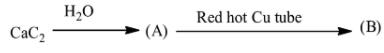 Product (B) of the reaction is:
Product (B) of the reaction is:
A.Toluene
B.Ethyl-benzene
C.Benzene
D.Butyne
Answer
592.2k+ views
Hint: We know that, calcium carbide $\left( {{\rm{Ca}}{{\rm{C}}_{\rm{2}}}} \right)$ is a compound which is used in industrial production of acetylene and cyanamide. Pure calcium carbide is colourless in nature.
Complete step by step answer:
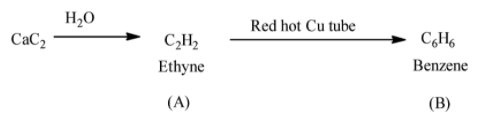
Now, we understand the reaction of calcium carbide and water. When calcium carbide reacts with water, calcium hydroxide and ethyne produce. The chemical reaction is shown as follows:
${\rm{Ca}}{{\rm{C}}_{\rm{2}}} + 2{{\rm{H}}_{\rm{2}}}{\rm{O}} \to {\rm{Ca}}{\left( {{\rm{OH}}} \right)_{\rm{2}}} + {{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{2}}}$
Ethyne undergoes cyclic polymerization. In cyclic polymerization, addition of alkyne molecules with each other produces a cyclic compound. Passing ethyne through a red hot copper tube at temperature of 873 K results in its cyclic polymerization. In this process, three ethyne molecules are required. Let’s write the chemical reaction of cyclic polymerization of ethyne.

From the above reaction it is clear that three molecules of ethyne undergo polymerization to form benzene when it passes through a red hot copper tube at 873 K. The triple bond of three ethyne molecules break down and rearrangement occurs which result in the formation of benzene.
Now, we summarize both the chemical reaction in a single reaction. The chemical reaction of calcium carbide with water produces ethyne (A) and when ethyne is passed through red hot copper tube benzene (B) produces.
Therefore, product B of the reaction is benzene. Hence, option C is correct.
Note: Polymerization is a process in which joining monomers results in polymers. There are two types of polymerization of ethyne, linear polymerization and cyclic polymerization. The cyclic polymerization of ethyne forms benzene and linear polymerization of molecules of ethyne forms polyethyne.
Complete step by step answer:
Now, we understand the reaction of calcium carbide and water. When calcium carbide reacts with water, calcium hydroxide and ethyne produce. The chemical reaction is shown as follows:
${\rm{Ca}}{{\rm{C}}_{\rm{2}}} + 2{{\rm{H}}_{\rm{2}}}{\rm{O}} \to {\rm{Ca}}{\left( {{\rm{OH}}} \right)_{\rm{2}}} + {{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{2}}}$
Ethyne undergoes cyclic polymerization. In cyclic polymerization, addition of alkyne molecules with each other produces a cyclic compound. Passing ethyne through a red hot copper tube at temperature of 873 K results in its cyclic polymerization. In this process, three ethyne molecules are required. Let’s write the chemical reaction of cyclic polymerization of ethyne.

From the above reaction it is clear that three molecules of ethyne undergo polymerization to form benzene when it passes through a red hot copper tube at 873 K. The triple bond of three ethyne molecules break down and rearrangement occurs which result in the formation of benzene.
Now, we summarize both the chemical reaction in a single reaction. The chemical reaction of calcium carbide with water produces ethyne (A) and when ethyne is passed through red hot copper tube benzene (B) produces.

Therefore, product B of the reaction is benzene. Hence, option C is correct.
Note: Polymerization is a process in which joining monomers results in polymers. There are two types of polymerization of ethyne, linear polymerization and cyclic polymerization. The cyclic polymerization of ethyne forms benzene and linear polymerization of molecules of ethyne forms polyethyne.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




