
Primary alkyl halide ${C_4}{H_9}Br$ (a) reacted with alcoholic $KOH$ to give compound (b). Compound (b) is reacted with $HBr$ to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives compound (d), ${C_8}{H_{18}}$ which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the formula of (a) and write the equations for all the reactions.
Answer
598.5k+ views
Hint: We have learned that there are two types of primary alkyl halides with the same molecular formula ${C_4}{H_9}Br$ that is n-butyl bromide and isobutyl bromide. So the first step is to find out which type of primary halide is used.
Complete answer:
In the problem it is given that when (a) is reacted with sodium metal it gives compound (d) , ${C_8}{H_{18}}$ which is different from the compound formed when n-butyl bromide is reacted with sodium hence now we now that the compound (a) is isobutyl bromide and compound (d ) is 2,5-methylhexane. So n-octane then 2,3-methyl hexane is obtained by the following reaction .
$2C{H_3}C{H_2}C{H_2}C{H_2}Br + 2Na \to C{H_3}C{H_2}C{H_2}C{H_2}C{H_2}C{H_2}C{H_2}C{H_3}$
$2C{H_3}CH(C{H_3})C{H_2}Br + 2Na \to C{H_3}CH(C{H_3})C{H_2}C{H_2}CH(C{H_3})C{H_3}$ ,
This reaction is also called the Wurtz reaction. Now take a look at this reaction which is when (a) reacted with alcohol $KOH$ to give compound (b) so we know that (a) is isobutyl bromide.
$C{H_3}CH(C{H_3})C{H_2}Br \to C{H_3}C(C{H_3}) = C{H_2}$
So here we see that the compound (b) is 2-methyl-1-propene.
Compound (b) is reacted with $HBr$ to give (c) which is an isomer of (a) ,now see this reaction and find out compound (c), it is important to remember that here product formation will be according to Markownikoff’s rule as the rule states that Hydrogen is added to the carbon which has the more number of hydrogen and the halide part is added to the carbon which has the least number of carbon. So the reaction is as follows:
$C{H_3}CH(C{H_3}) = C{H_2} \to C{H_3} - CBr(C{H_3})C{H_3}$
Here the product is tert- butyl bromide which is an isomer of isobutyl bromide. Structural formula of isobutyl is shown in figure:
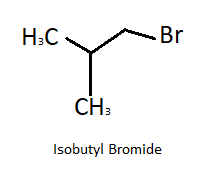
Note: In this sequence of reactions first we have identified the compound (a) which is iso butylbromide, then we write each reaction as it was given in the problem in order to get all the compounds.
Complete answer:
In the problem it is given that when (a) is reacted with sodium metal it gives compound (d) , ${C_8}{H_{18}}$ which is different from the compound formed when n-butyl bromide is reacted with sodium hence now we now that the compound (a) is isobutyl bromide and compound (d ) is 2,5-methylhexane. So n-octane then 2,3-methyl hexane is obtained by the following reaction .
$2C{H_3}C{H_2}C{H_2}C{H_2}Br + 2Na \to C{H_3}C{H_2}C{H_2}C{H_2}C{H_2}C{H_2}C{H_2}C{H_3}$
$2C{H_3}CH(C{H_3})C{H_2}Br + 2Na \to C{H_3}CH(C{H_3})C{H_2}C{H_2}CH(C{H_3})C{H_3}$ ,
This reaction is also called the Wurtz reaction. Now take a look at this reaction which is when (a) reacted with alcohol $KOH$ to give compound (b) so we know that (a) is isobutyl bromide.
$C{H_3}CH(C{H_3})C{H_2}Br \to C{H_3}C(C{H_3}) = C{H_2}$
So here we see that the compound (b) is 2-methyl-1-propene.
Compound (b) is reacted with $HBr$ to give (c) which is an isomer of (a) ,now see this reaction and find out compound (c), it is important to remember that here product formation will be according to Markownikoff’s rule as the rule states that Hydrogen is added to the carbon which has the more number of hydrogen and the halide part is added to the carbon which has the least number of carbon. So the reaction is as follows:
$C{H_3}CH(C{H_3}) = C{H_2} \to C{H_3} - CBr(C{H_3})C{H_3}$
Here the product is tert- butyl bromide which is an isomer of isobutyl bromide. Structural formula of isobutyl is shown in figure:

Note: In this sequence of reactions first we have identified the compound (a) which is iso butylbromide, then we write each reaction as it was given in the problem in order to get all the compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




