
How will you prepare the following compounds using Grignard reagent?
(i) Primary alcohol
(ii) Secondary alcohol
Answer
599.7k+ views
Hint:
To prepare alcohols by Grignard reagent, we need a reactant which has a carbonyl group. Grignard reagent adds an alkyl group into the reactant compound because it has nucleophilic alkyl group attached with Magnesium metal.
Complete answer:
- RMgX is the common formula of a Grignard reagent.
- Here, nucleophilic alkyl groups attack the carbonyl carbon and alcohol is formed upon hydrolysis.
- Only in the case if we use Formaldehyde as a starting material, then only we will get primary alcohol as a product. Let’s see why this happens.
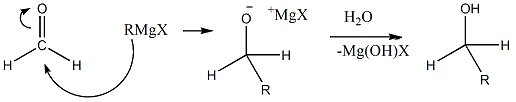
- We can see that if one more alkyl group was attached to the starting material, then secondary alcohol would have been formed. So, this is the only starting material from which we can obtain primary alcohol.
- If we take any other aldehyde and then allow it to react with Grignard reagent, then always secondary aldehyde will be formed. Let’s see the reaction.
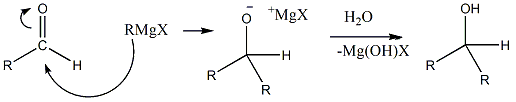
- We can take any alkyl group as R in the Grignard reagent. As a halide, Chlorine is preferred in Grignard reagent as it reacts easily and gives desired products with good yield. Other halides can also be used but it is often less seen.
Additional Information:
- Grignard reagent is a very reactive reagent and can react with atmospheric moisture and Carbon dioxide as well. So, care is needed to be taken to handle Grignard reagents.
- Grignard reagent is an organometallic reagent as it contains Magnesium metal in its structure. Magnesium is present in +2 oxidation state and carbon that is directly attached with Magnesium metal is having negative charge.
- In order to get the final product, treatment with water (Hydrolysis) is necessary.
Note:
- As Formaldehyde contains two Hydrogen atoms directly bonded with the carbonyl carbon, it will give different types of alcohol which is an exception among all the aldehydes that needs to be remembered. Grignard reagents do not attack on the carbon that is only bearing hydroxyl groups as it is not much electrophilic.
To prepare alcohols by Grignard reagent, we need a reactant which has a carbonyl group. Grignard reagent adds an alkyl group into the reactant compound because it has nucleophilic alkyl group attached with Magnesium metal.
Complete answer:
- RMgX is the common formula of a Grignard reagent.
- Here, nucleophilic alkyl groups attack the carbonyl carbon and alcohol is formed upon hydrolysis.
- Only in the case if we use Formaldehyde as a starting material, then only we will get primary alcohol as a product. Let’s see why this happens.

- We can see that if one more alkyl group was attached to the starting material, then secondary alcohol would have been formed. So, this is the only starting material from which we can obtain primary alcohol.
- If we take any other aldehyde and then allow it to react with Grignard reagent, then always secondary aldehyde will be formed. Let’s see the reaction.

- We can take any alkyl group as R in the Grignard reagent. As a halide, Chlorine is preferred in Grignard reagent as it reacts easily and gives desired products with good yield. Other halides can also be used but it is often less seen.
Additional Information:
- Grignard reagent is a very reactive reagent and can react with atmospheric moisture and Carbon dioxide as well. So, care is needed to be taken to handle Grignard reagents.
- Grignard reagent is an organometallic reagent as it contains Magnesium metal in its structure. Magnesium is present in +2 oxidation state and carbon that is directly attached with Magnesium metal is having negative charge.
- In order to get the final product, treatment with water (Hydrolysis) is necessary.
Note:
- As Formaldehyde contains two Hydrogen atoms directly bonded with the carbonyl carbon, it will give different types of alcohol which is an exception among all the aldehydes that needs to be remembered. Grignard reagents do not attack on the carbon that is only bearing hydroxyl groups as it is not much electrophilic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




