
How will you prepare benzyl alcohol from toluene?
Answer
599.7k+ views
Hint: Toluene contains a methyl group attached to a phenyl group. Benzyl alcohol contains a hydroxymethyl group attached to a phenyl group. First replace one hydrogen atom of methyl group of toluene with a chlorine atom. Then replace the chlorine atom with a hydroxyl group.
Complete answer:
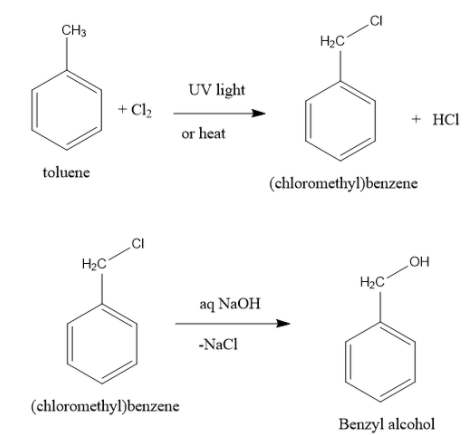
In toluene, a methyl group is attached to a benzene ring. In benzyl alcohol, one hydroxyl group is attached to the benzyl group. A benzyl group is a combination of methylene group with phenyl group.
The conversion of toluene into benzyl alcohol can be achieved in two steps.
Free radical chlorination of toluene with chlorine in presence of ultraviolet light or heat give benzyl chloride. One hydrogen atom of methyl group of toluene is replaced with chlorine atom. A molecule of hydrogen chloride is eliminated. In this reaction, the benzene ring remains as it is. No reaction occurs between benzene ring and chlorine. Only the aliphatic methyl group reacts with chlorine.
In the next step, benzyl chloride undergoes nucleophilic substitution reaction in presence of aqueous sodium hydroxide solution to form benzyl alcohol. A molecule of sodium chloride is eliminated.
Note: Alkyl halides react with aqueous sodium hydroxide to undergo substitution reaction, in which halogen atom is replaced with hydroxyl group. If instead of aqueous sodium chloride, the reagent used is alcoholic sodium hydroxide, then alkyl halides prefer dehydrohalogenation reaction.
Complete answer:
In toluene, a methyl group is attached to a benzene ring. In benzyl alcohol, one hydroxyl group is attached to the benzyl group. A benzyl group is a combination of methylene group with phenyl group.
The conversion of toluene into benzyl alcohol can be achieved in two steps.
Free radical chlorination of toluene with chlorine in presence of ultraviolet light or heat give benzyl chloride. One hydrogen atom of methyl group of toluene is replaced with chlorine atom. A molecule of hydrogen chloride is eliminated. In this reaction, the benzene ring remains as it is. No reaction occurs between benzene ring and chlorine. Only the aliphatic methyl group reacts with chlorine.
In the next step, benzyl chloride undergoes nucleophilic substitution reaction in presence of aqueous sodium hydroxide solution to form benzyl alcohol. A molecule of sodium chloride is eliminated.

Note: Alkyl halides react with aqueous sodium hydroxide to undergo substitution reaction, in which halogen atom is replaced with hydroxyl group. If instead of aqueous sodium chloride, the reagent used is alcoholic sodium hydroxide, then alkyl halides prefer dehydrohalogenation reaction.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




