
P-P bond is present in:
(A) ${{P}_{4}}{{O}_{10}}$
(B) ${{P}_{4}}{{O}_{6}}$
(C) ${{H}_{4}}{{P}_{2}}{{O}_{6}}$
(D) ${{H}_{4}}{{P}_{2}}{{O}_{7}}$
Answer
573.9k+ views
Hint: P-P bond means that the two phosphorous atoms are joined with each other which leads to formation of a bridge between the molecules. P-P is a covalent bond which is formed by the sharing of electrons by different atoms which are present in the molecule.
Complete step by step solution:
Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ , in this hypo means beneath or less than. The Hypophosphoric acid has an oxidation state of +4 between the phosphorus acid and the phosphoric acid.
The oxidation state of P in${{P}_{4}}{{O}_{10}}$, ${{P}_{4}}{{O}_{6}}$ and ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ is +5. The calculated oxidation state is equal to the maximum oxidation state of phosphorus hence there is P-O-P bond present in all these compounds.
Among the given options Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ forms a P-P double bond because the oxidation state if +4 which is less than the maximum oxidation state of P. and when the calculated oxidation state is less than the maximum oxidation state then there is a direct linkage between the elements here it is P-P linkage.
The structure of Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is shown below:
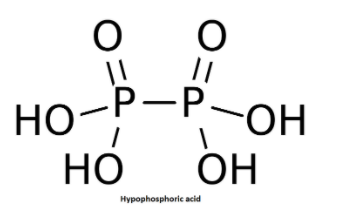
In this structure we can note that there are two P-O bonds having bond length of 151 pm and four P-OH bonds having bond length of 151 pm and one P-P single bond having bond length of 219 pm.
Hence the correct answer is option ©
Note: Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is used as a bleaching agent. It is also used as a reducing agent, as a stimulant and pharmaceutical agent and also as a wetting agent. Hypophosphoric acid is a mineral acid which is present as dehydrate in the solid state.
Complete step by step solution:
Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ , in this hypo means beneath or less than. The Hypophosphoric acid has an oxidation state of +4 between the phosphorus acid and the phosphoric acid.
The oxidation state of P in${{P}_{4}}{{O}_{10}}$, ${{P}_{4}}{{O}_{6}}$ and ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ is +5. The calculated oxidation state is equal to the maximum oxidation state of phosphorus hence there is P-O-P bond present in all these compounds.
Among the given options Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ forms a P-P double bond because the oxidation state if +4 which is less than the maximum oxidation state of P. and when the calculated oxidation state is less than the maximum oxidation state then there is a direct linkage between the elements here it is P-P linkage.
The structure of Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is shown below:

In this structure we can note that there are two P-O bonds having bond length of 151 pm and four P-OH bonds having bond length of 151 pm and one P-P single bond having bond length of 219 pm.
Hence the correct answer is option ©
Note: Hypophosphoric acid i.e. ${{H}_{4}}{{P}_{2}}{{O}_{6}}$ is used as a bleaching agent. It is also used as a reducing agent, as a stimulant and pharmaceutical agent and also as a wetting agent. Hypophosphoric acid is a mineral acid which is present as dehydrate in the solid state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




