
How many position isomers of dibromo naphthalene are possible if each ring naphthalene has one halogen ?
Answer
592.5k+ views
Hint: The position isomer can be defined as the isomer which differs in position of the substituted atom in the chain or ring. The number of position isomers possible for naphthalene is equal to the number of hydrogen atoms present on the ring or we can say the number of replaceable hydrogen atoms present.
Complete step by step answer:
First, let us understand the structure of the compound given to us. We have dibromo i.e. two bromine atoms are there and a naphthalene ring is present. Further, it is said that each ring has one halogen i.e. one bromine atom.
We already know that naphthalene consists of two benzene rings that are fused. These rings are resonance stabilised and have many properties similar to benzene.
Each ring in naphthalene consists of one bromine atom substituted at any place.
The position isomers of dibromo naphthalene can be drawn as follows-
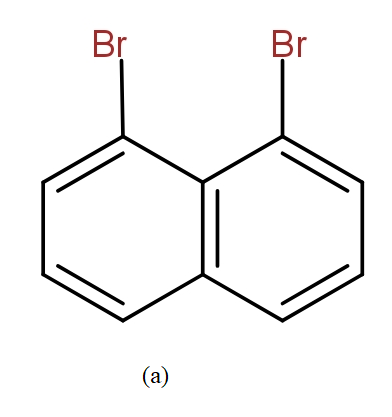
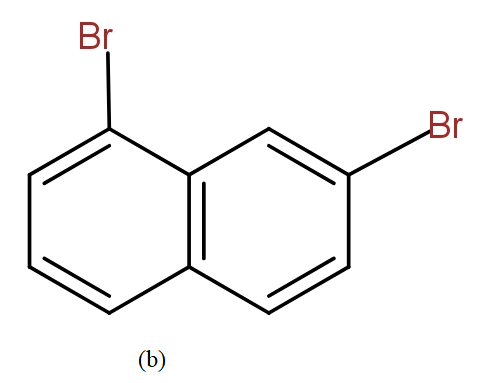
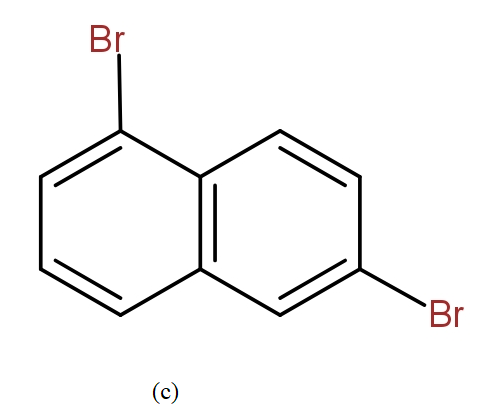
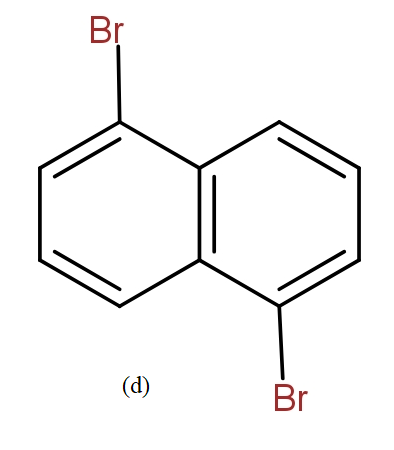

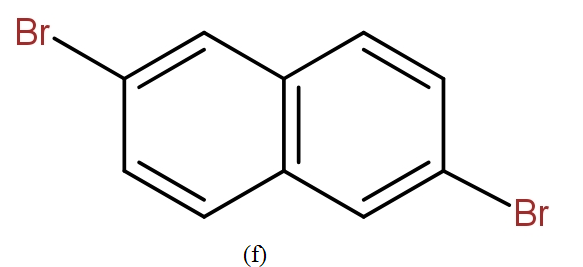

Thus, we can say that no more structure could be drawn.
So, the total number of position isomers of dibromo naphthalene are possible if each ring naphthalene has one bromine atom are 7.
Note: It must be noted that we can not say that the following structures could also be drawn and these are position isomers for dibromo naphthalene.
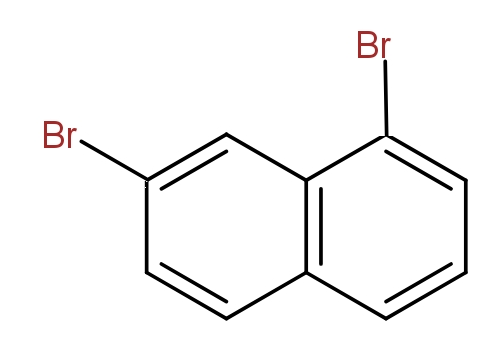
This is because this is the same as the structure (b) if we start numbering from the other side.
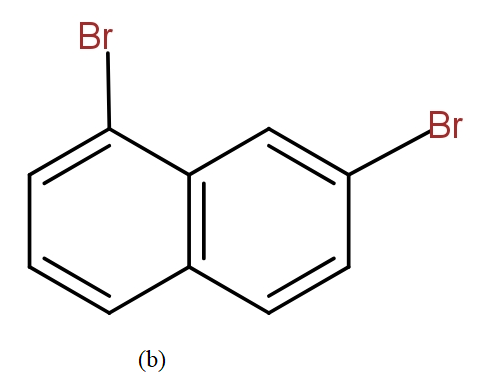
Thus, even other structures that could be drawn yield the same IUPAC name. So, they are not counted as different position isomers.
Complete step by step answer:
First, let us understand the structure of the compound given to us. We have dibromo i.e. two bromine atoms are there and a naphthalene ring is present. Further, it is said that each ring has one halogen i.e. one bromine atom.
We already know that naphthalene consists of two benzene rings that are fused. These rings are resonance stabilised and have many properties similar to benzene.
Each ring in naphthalene consists of one bromine atom substituted at any place.
The position isomers of dibromo naphthalene can be drawn as follows-







Thus, we can say that no more structure could be drawn.
So, the total number of position isomers of dibromo naphthalene are possible if each ring naphthalene has one bromine atom are 7.
Note: It must be noted that we can not say that the following structures could also be drawn and these are position isomers for dibromo naphthalene.

This is because this is the same as the structure (b) if we start numbering from the other side.

Thus, even other structures that could be drawn yield the same IUPAC name. So, they are not counted as different position isomers.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




