
How many P-O-P bonds are present in cyclic meta-phosphoric acid? Also, give it structure.
Answer
588.6k+ views
Hint: Molecular formula of cyclic meta-phosphoric acid is $({H_3}{O_9}{P_3})$. To find the number of P-O-P bonds in cyclic meta-phosphoric acid, we need to refer to its structure and then count the number of P-O-P bonds.
Complete step by step solution:
First, let us draw the structure of cyclic meta-phosphoric acid$({H_3}{O_9}{P_3})$.
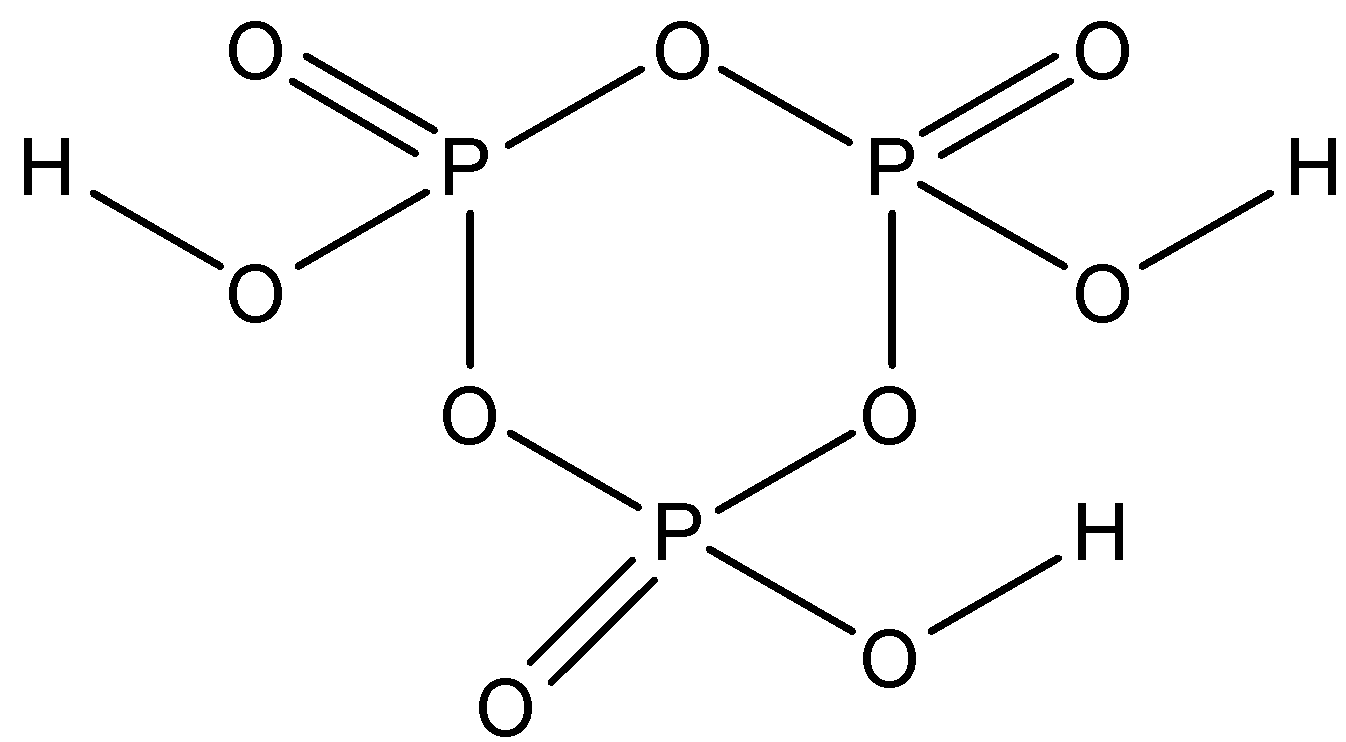
Now as we can see in the above structure, there are 3 types of bonds:$P = O$ ,$P - O - P$ and $O - H$ bonds. We have been given to find the number of P-O-P bonds, so count the number of P-O-P bonds in the above drawn structure.
It is clear from the structure that there are three P-O-P bonds in the structure of cyclic meta-phosphoric acid. Hence, this is the required answer.
Additional information: Cyclic meta-phosphoric acid is $({H_3}{O_9}{P_3})$is also known as cyclo triphosphate acid, trimetaphosphoric acid, and trimetaphosphate. It belongs to the class of inorganic compounds called non-metal phosphates. Cyclic meta-phosphoric acid is the cyclic anhydride of triphosphoric acid which is $({H_5}{P_3}{O_{10}})$ while ATP (Adenosine triphosphate) are the esters of triphosphoric acid.
Note: You can also be asked about the total number of sigma and pi-bonds in the cyclic meta-phosphoric acid. So, there are a total of fifteen bonds in the structure of cyclic meta-phosphoric acid and out of these fifteen bonds, there are twelve sigma bonds ($\sigma $) and three pi-bonds ($\pi $).
Complete step by step solution:
First, let us draw the structure of cyclic meta-phosphoric acid$({H_3}{O_9}{P_3})$.

Now as we can see in the above structure, there are 3 types of bonds:$P = O$ ,$P - O - P$ and $O - H$ bonds. We have been given to find the number of P-O-P bonds, so count the number of P-O-P bonds in the above drawn structure.
It is clear from the structure that there are three P-O-P bonds in the structure of cyclic meta-phosphoric acid. Hence, this is the required answer.
Additional information: Cyclic meta-phosphoric acid is $({H_3}{O_9}{P_3})$is also known as cyclo triphosphate acid, trimetaphosphoric acid, and trimetaphosphate. It belongs to the class of inorganic compounds called non-metal phosphates. Cyclic meta-phosphoric acid is the cyclic anhydride of triphosphoric acid which is $({H_5}{P_3}{O_{10}})$ while ATP (Adenosine triphosphate) are the esters of triphosphoric acid.
Note: You can also be asked about the total number of sigma and pi-bonds in the cyclic meta-phosphoric acid. So, there are a total of fifteen bonds in the structure of cyclic meta-phosphoric acid and out of these fifteen bonds, there are twelve sigma bonds ($\sigma $) and three pi-bonds ($\pi $).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




